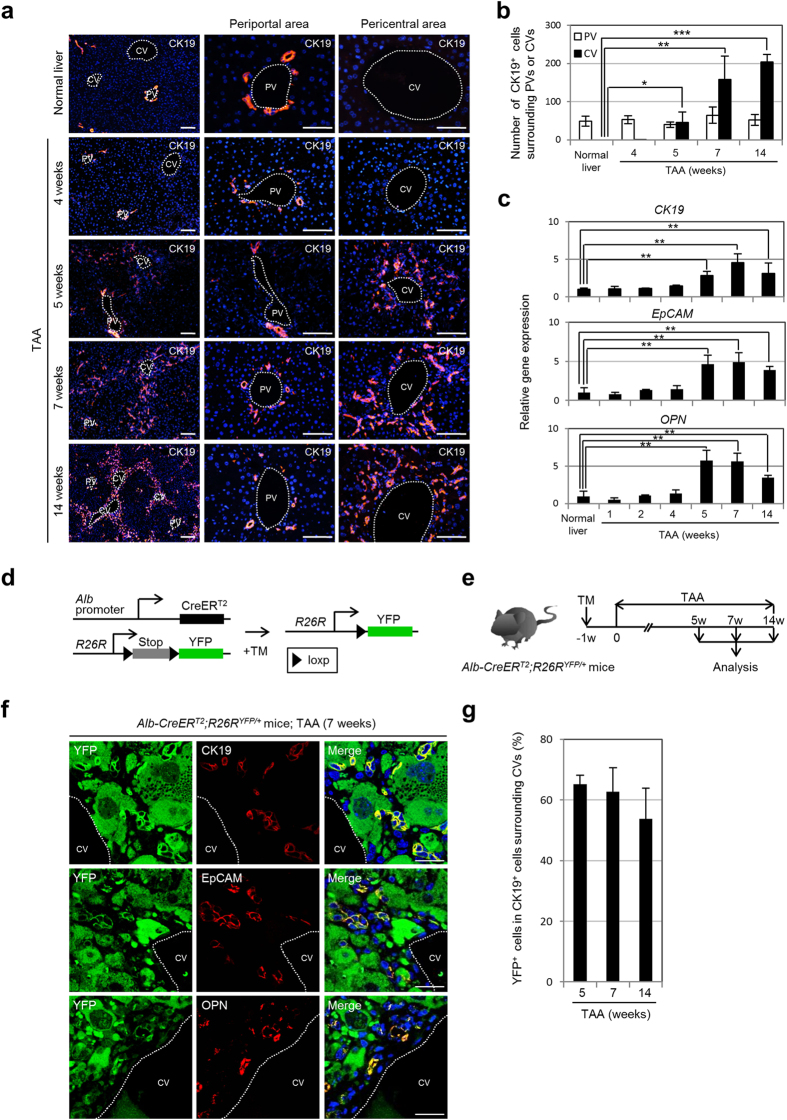
Figure 1. Majority of TAA-induced pericentral biliary lineage cells arise from hepatocytes.
(a) Immunofluorescence staining of CK19 in the livers of normal and TAA-administered mice. (b) Numbers of CK19-positive cells surrounding the PVs or CVs in the livers of normal and TAA-administered mice (25 PVs and 25 CVs per mouse were analyzed in discontinuous liver sections from 3–4 different liver lobes). The data represent means ± SD (n = 3). (c) RT-qPCR analyses of CK19, EpCAM, and OPN expression were carried out using total RNA derived from the livers of normal and TAA-administered mice. All data were normalized by the value for the internal control gene Gapdh and expressed as fold differences from the value in the normal liver. The data represent means ± SD (n = 3). (d) Experimental procedure to follow the lineage of hepatocytes in the mouse liver. In the presence of TM, CreERT2 expressed from the Alb genomic locus translocates into the nucleus and removes the loxP-flanked stop cassette from the R26R allele, leading to permanent heritable expression of the YFP gene. (e) Experimental procedure to induce biliary lineage cells around the CVs in the liver. Alb-CreERT2;R26RYFP/+ mice were administered TAA from 1 week after TM injection, and the liver tissues were analyzed after 5, 7, and 14 weeks of TAA administration. w, week(s). (f) Co-immunofluorescence staining of YFP with CK19, EpCAM, or OPN in the liver of Alb-CreERT2;R26RYFP/+ mice after 7 weeks of TAA administration. (g) Percentages of CK19-positive biliary lineage cells co-expressing YFP around the CVs in the liver of Alb-CreERT2;R26RYFP/+ mice after 5, 7, and 14 weeks of TAA administration (10 CVs per mouse were analyzed in discontinuous liver sections from 3–4 different liver lobes). The data represent means ± SD (n = 3). DNA was stained with DAPI. Scale bars: 100 μm (a) and 25 μm (f). *P < 0.05. **P < 0.01. ***P < 0.001.