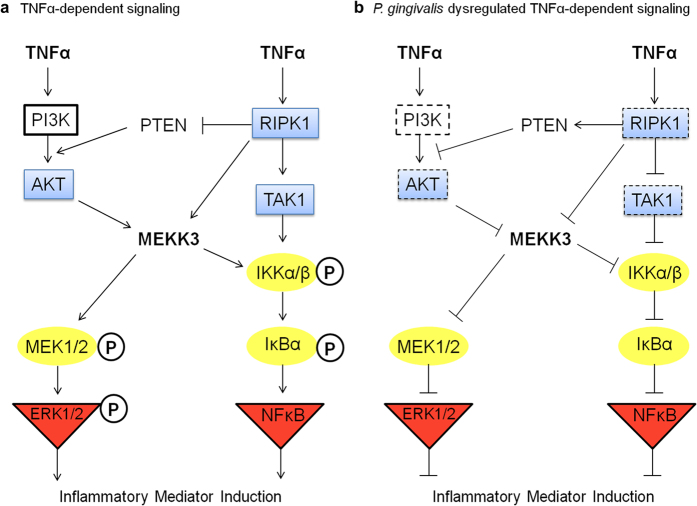
Figure 7. Model of P. gingivalis-induced attenuation of TNFα-dependent innate immune signaling responses.
(a) Normal TNFα-dependent signal propagation is capable of proceeding through both AKT and RIPK1-dependent pathways, which culminates in the activation of either ERK1/2 or NFκB and thus promotes inflammatory mediator induction. RIPK1 and AKT can both interact with MEKK3, providing cross-talk for signal flux between these pathways. Additionally, RIPK1 indirectly activates AKT via its role in reducing expression of PTEN, a phosphatase that inhibits the PI3K/AKT pathway. During TNFα stimulation, signal flux can be monitored through these pathways by the detection of high phosphorylation status of MEK1/2, IKKα/β and IκBα. (b) Here we demonstrated that P. gingivalis dysregulates TNFα-dependent signaling via degradation of host cell RIPK1, TAK1 and AKT kinases. Degradation of host kinases would limit the required interactions of RIPK1 and AKT with MEKK3, in addition to IKK recruitment, effectively blunting ERK1/2 and NFκB activation and proinflammatory induction. Cross-talk between pathways would be reduced due to a lack of RIPK1 initiating an increase in PTEN levels, allowing for inhibition of AKT activity. Attenuated signal flux was observed within downstream signaling proteins (reduced phosphorylation of MEK1/2, IKKα/β and IκBα). Of note, Nakayama et. al. have reported that P. gingivalis infection impairs PI3K/AKT activity through degradation of an unidentified membrane protein, creating a secondary assault to the signaling capacity of the AKT pathway. Dashed boxes represent host proteins whose protein abundance and/or activity are disrupted by P. gingivalis. Circled proteins are internal signaling proteins whose phosphorylation status are reduced and those in triangles are terminal signaling proteins identified to have reduced phosphorylation and/or activity during P. gingivalis induced dysregulated TNFα signaling.