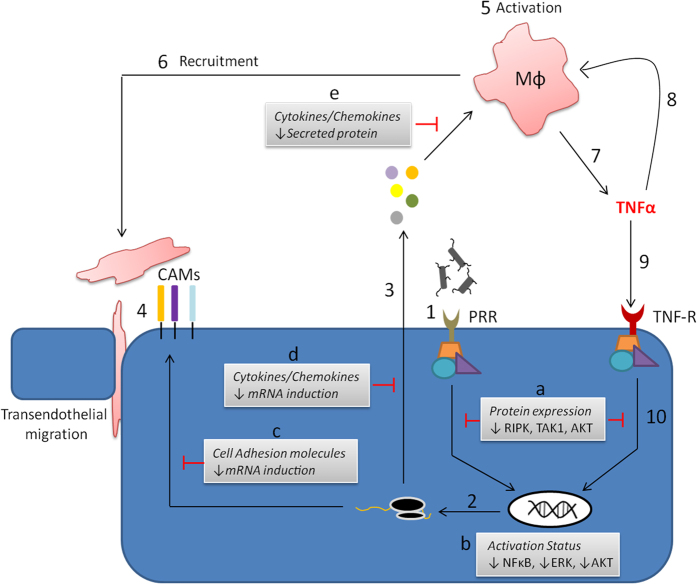
Figure 8. Model of P. gingivalis gingipain-driven subversion of innate immunity at the endothelium.
Immune activation of the endothelium during microbial infection involves a number of critical events that are influential in facilitating resolution of infection. (1). Recognition of pathogens by the endothelium occurs through pattern recognition receptors, effectively driving specific genetic programs, leading to mRNA induction (2) and endothelial activation. This is primarily observed as secretion of cytokines and recruitment factors (3), in addition to the up regulation of cell adhesion molecules (4). Inflammatory mediator output activates (5) and recruits (6) immune cells to the site of the infection in order to help mediate clearance and recovery of the tissue, while cell adhesion molecules allow for enhanced transendothelial migration of professional immune cells into deeper tissues. Recruited inflammatory cells can produce large amounts of TNFα (7), which can act in an autocrine (8) or paracrine fashion (9). Endothelial responsiveness to TNFα functions as an amplification step to further activate the endothelium itself (10), while also promoting increased immune cell recruitment and/or activation due to further increases in inflammatory mediator output. We have demonstrated that P. gingivalis gingipains modulate endothelial innate immunity at several key points. Degradation of RIPK1, TAK1 and AKT kinases (a) results in attenuated responsiveness to TNFα. This was observed as reduced activation of NFκB, ERK and AKT pathways (b), in addition to impaired mRNA induction of both cell adhesion molecules (c) and secreted cytokines and chemokines (d). Analogous to TNFα-dependent responses, we propose that host immune kinase expressional changes (a) mediate reduced mRNA induction downstream of PRR-dependent responses to P. gingivalis. Significantly, as opposed to simply subverting the ability of the endothelial cell to properly induce inflammatory mediator secretion, gingipain activity can directly degrade already produced and secreted effectors (e).