Abstract
Objectives
To evaluate the effect of low-level laser therapy on healing of extracted tooth socket of healthy rabbits.
Design
The sample of this study was 20 male rabbits of 2–2.5 kg weight with age range of 8–12 months. Right and left lower first premolar teeth were extracted. The extraction sockets of lower right first premolar were irradiated with 0.9 W gallium-aluminum-arsenide (GaAlAs) diode laser for 5 min, immediately after extraction and then every 72 h for the next 12 days. The extraction socket of left side were not exposed to laser and served as a control. The animals were sacrificed after 7, 14, 30 and 45 days and the experimental and control sockets were removed from the harvested mandibles and prepared for haematoxylin and eosin staining and Masson’s stain. The prepared slides were examined under light microscope for histological and histomorphometric examination.
Results
The histological examination showed that diode laser-treated sockets demonstrated early formed new bone with faster maturation of primary bone to secondary bone as compared to non-treated control sockets. Histomorphometric analysis revealed a statistically significant increase in the density and volume of trabecular bone in laser-treated sockets than control sockets.
Conclusion
Diode laser application to tooth extraction socket has a positive effect on bone formation.
Keywords: Diode laser, Healing, Rabbit, Tooth socket
Introduction
Wounds involving the oral mucosa and jaw bones are commonly encountered in oral surgical practice. Tooth extraction is the most common surgical procedure performed by dentists. Healing of such wounds is straight forward in the majority of cases; however, some wounds may take long time to heal and are associated with great discomfort to the patient.
One of the most important and common complications following tooth extraction is dry socket. This phenomenon occurs when a blood clot dissolves and consequently, the exposure of alveolar bone happens. Dry socket is marked by severe and progressive pain, halitosis, regional lymphadenitis, and activity reduction. It is mostly prevalent in surgical extraction of mandibular third molar [1]. These complications reach their maximal at 12–48 h after surgery, but may completely resolve in 5–7 days [2].
Experimental research has elucidated several methods to hasten bone regeneration for defects and fractures of bone, including mechanical stimulation [3, 4], electromagnetic fields [5, 6], low-intensity ultrasound [7, 8], bioactive materials [9], biological growth factors [10], and low-level laser therapy (LLLT) [11, 12].
Low-level laser therapy (LLLT) is also known as “soft laser therapy” and bio-stimulation. The use of LLLT in health care has been documented in the literature for more than three decades. Numerous research studies have demonstrated that LLLT is effective for some specific applications in dentistry [13]. LLLT has been used with various power densities to stimulate wound healing. Laser energy could enhance osteoblastic proliferation, collagen synthesis by fibroblast, activation of lymphatic system, proliferation of epithelial cells and fibroblast, increased angiogenesis and bone formation [14]. Park and Kang [15] demonstrated that 980-nm gallium-aluminum-arsenide (GaAlAs) low-intensity diode laser irradiation is beneficial for the initial stages of alveolar bone healing of tooth extraction socket and for further calcification in both diabetic and normal rats when applied every day at a dose of 13.95 J/cm2 for 60 s.
One of the mechanisms thought to be responsible for the physiologic effect of laser therapy is the ability of laser photons to alter cellular metabolism as a result after its absorption by cytochrome C oxidase. As a result of this stimulation of the respiratory chain in the mitochondria, adenosine triphosphate (ATP) production is increased [16]. It is postulated that this increased ATP production and consequent energy source for the cellular machinery allows for increased function and metabolism of cells in tissues that are ischemic, wounded, intoxicated, or poorly perfused [17].
The outcome of LLLT treatment varies with the treatment parameters including: power, power density, wavelength, beam profile, energy, energy density, number and frequency of treatment and duration of treatment [18].
The purpose of this study was to evaluate effect of laser therapy in acceleration of bone healing in extracted tooth socket of healthy rabbits.
Materials and Methods
The laser device that was utilized in the present study is gallium aluminum arsenide diode laser (A.R.C. laser GmbH, Germany). Laser parameters were set as follows: wavelength, 808 nm; output, 0.9 W; dose, 1459 J/cm2; continuous radiation mode.
Twenty healthy local rabbits were used in the present study. The average weight of the animals was 2–2.5 kg and the age was 8–12 months. The animals were housed in a large well ventilated room. The animals were exposed to alternative cycle of 12 h light and 12 h dark photoperiod at 24–28 °C with 55–70 % humidity. The animals were fed the same type of food which included carrots, dry bread, wheat, lettuce and radish. Tap water was also provided add libitum. All measurements were taken to prevent animal cruelty and the study was conducted in line with the national and international guidelines for using experimental animals for medical research, and has been approved by the ethics committee of the University of Duhok. Animals used in this research received every consideration for their comfort; they were properly housed, fed, and their surroundings kept in a sanitary condition.
All animals were weighted to calculate the dose of anesthesia. General anaesthesia of the animals was achieved by intramuscular injection of (5 mg/kg) xylazine (Alfasan, Holland) in combination with 35 mg/kg ketamine (Gracure Pharmaceutical Ltd., India). The animals were anaesthetized before starting surgical procedure and also before each laser application.
After the onset of anaesthesia, which usually occurred in 10 min, the right and left first premolar teeth were extracted. Straight elevator was used to luxate the tooth, and once loosened the tooth was gently rotated and pressed back into the socket to destroy apical germinal tissue. Failure to do this may result in tooth re-growth. Finally the tooth was removed by movement in lingual direction.
Laser beam was applied for 5 min, immediately after extraction and every 72 h for the next 12 days. The probe was placed in contact with the socket of the right side in perpendicular direction and in rotation movement so that all walls of the socket will be lasered. The socket of the left side was not exposed to the laser and served as control.
Euthanasia of the animals was achieved under anaesthesia at four time intervals. Five rabbits each were sacrificed at 7, 14, 30 and 45 days. The mandible was harvested and the overlying soft tissue was removed with scalpel. The experimental and control sockets with a rim of surrounding bone was removed with saw and the specimens were placed in 10 % formalin for fixation. The specimens were decalcified by nitric acid 5 % for 3–5 days and then dehydrated by ethanol alcohol and embedded in paraffin. Coronal histological sections were prepared of 5 µm thickness and stained with haematoxylin and eosin for routine examination and with Masson’s trichrome for bone trabeculae detection.
All the morphometric analysis was done in blinded way and by image J software program (NIH, Betheseda, USA). Density of trabecular bone in mm was calculated in 40 randomly selected histological fields for each laser and non-laser tooth sockets captured by digital camera connected to the Nikon microscope taken at 10 magnifications. The percentage of volume density of trabecular bone was calculated for both groups by dividing volume density of each trabecular bone to the total area and compared statistically by student t test. A P value <0.05 was considered as significant.
Results
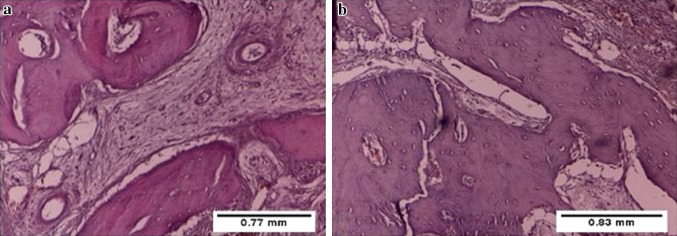
After 7 days the non-lasered side showed cellular granulation tissue formation with hemorrhagic areas and starting of primary (woven) bone formation, which by this time was thin and occupied small areas, and infiltration of inflammatory cells and less amount of bone marrow (Fig. 1). The lasered sockets showed mature trabecular bone formation, fibrosis, more osteocytes proliferation within trabecular bone and more active osteoblasts around trabecular bone than non-lasered socket. In addition, new blood vessels and more fibrous granulation tissue were present (Figs. 2, 3).
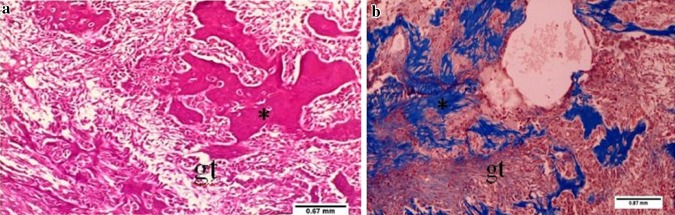
Fig. 1.
Photomicrograph of tooth socket after 7 days of extraction without laser treatment showing cellular granulation tissue (gt), haemorrhage, infiltration of inflammatory cells, newly formed bone (asterisk) with less amount of bone marrow. a H&E ×10, b Masson’s trichrome ×10
Fig. 2.
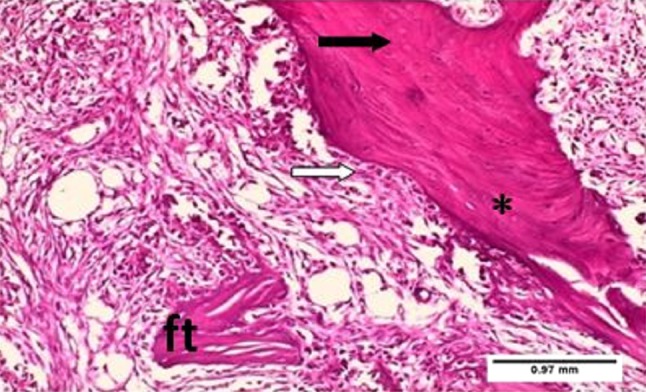
Photomicrograph of tooth socket after 7 days of extraction with laser treatment showing fibrous tissue (ft), newly formed trabecular bone (asterisk),massive infiltration of active osteoblasts (white arrow) and osteocytes (black arrow). H&E ×10
Fig. 3.
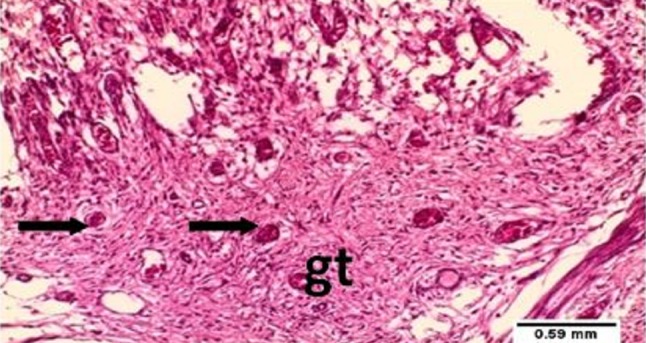
Photomicrograph of tooth socket after 7 days of extraction with laser treatment showing newly formed blood vessels (arrows) and fibrous granulation tissue (gt). H&E ×10
At 14 days there was scanty new irregular bone trabeculae in the non lasered sockets. The lasered sockets showed mature regular bone trabeculae, thicker and longer than the non-lasered sockets. In addition, the lasered sockets revealed bone morrow compartment much narrower than the non-lasered sockets with the bone matrix in the lasered sockets arranged in a manner similar to the aspect of a system of Havers (Fig. 4).
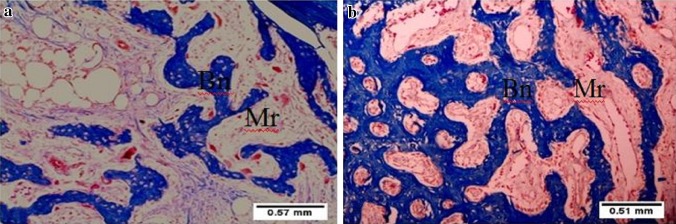
Fig. 4.
Photomicrograph of tooth socket after 14 days of extraction. a non-lasered sockets showing newly formed irregular bone trabeculae (Bn) and abundant bone marrow spaces (Mr). b Lasered sockets showing mature completely formed bone trabeculae (Bn) and scanty bone marrow spaces (Mr). Masson’s trichrome ×10
At 30 days, secondary mature trabecular bone was more observed in the lasered socket than non lasered socket that was still immature and irregular (Fig. 5).
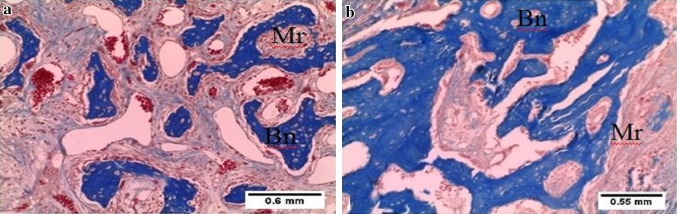
Fig. 5.
Photomicrograph of tooth socket after 30 days of extraction. a Non-lasered sockets showing immature bone trabeculae (Bn) and abundant bone marrow spaces (Mr). b Lasered sockets showing abundant mature bone trabeculae (Bn) and few bone marrow spaces (Mr). Masson’s trichrome ×10
After 45 days, osteon formation was more observed on lasered socket that is functional units of much compact bone appeared as cylindrical structure of lamellae and osteocytes around central canal (Haversian canal) which contains blood vessels and nerves. The non-lasered sockets still demonstrate less bone trabeculae that remain immature and irregular (Figs. 6, 7).
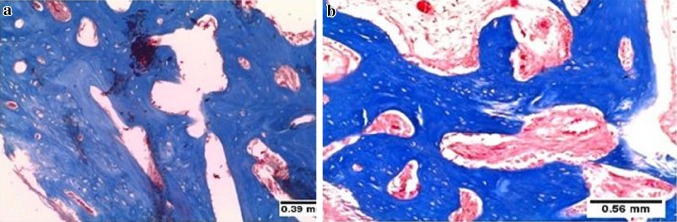
Fig. 6.
Photomicrograph of tooth socket after 45 days of extraction. a Lasered socket showing compact bone with osteon formation. b Non-lasered sockets showing still immature and irregular trabecular bone. Masson’s trichrome ×10
Fig. 7.
Photomicrograph of tooth socket after 45 days of extraction showing irregular immature bone of non-lasered sockets (a) as compared to more compact trabecular bone formation in the lasered sockets (b). H&E ×10
At 14 and 30 days after extraction, the histomorphometric analysis showed that trabecular bone volume density is significantly greater in lasered sockets than non-lasered sockets (P < 0.05) (Table 1).
Table 1.
Trabecular bone volume density (per mm) in lasered and non-lasered sockets after 14 and 30 days of tooth extraction
| Days post-extraction | Groups | No. | Trabecular bone volume density/mm | P value |
|---|---|---|---|---|
| 14 days | Lasered sockets | 5 | 1.84 ± 0.23 | <0.05* |
| Non-lasered sockets | 5 | 1.52 ± 0.35 | ||
| 30 days | Lasered sockets | 5 | 2.08 ± 0.28 | <0.05* |
| Non-lasered sockets | 5 | 1.61 ± 0.34 |
* Significant
Discussion
This study attempts to assess the effect of diode in ameliorating bone repair in tooth extraction sockets of rabbits. The rabbit model has the advantage that it is easy to handle the animals compared with rats, mice. Small rodents have primitive bone structures and do not have Haversian systems [19] and although little is known about the importance of this anatomical difference between rodents and humans, this makes bone repair in these animals different from that seen in human beings. Rabbits have Haversian systems that are identical to that of human being, which is an important advantage in terms of extrapolation of results obtained with such animals for human bone repair. However, the rapid healing process in these animals compared with humans, make them valuable bioassay for screening of comparable technologies, but questionable for direct transfer of results to the human clinical situation [19]. There is a consensus that the cycle of rabbit bone repair is completed in approximately 42 days [20]. Therefore, the evaluation carried out in periods of 7, 21 and 42 days allowed for the analysis of the initial, intermediate and final stages of bone repair [20, 21].
Histological results of the present study indicated that diode laser ameliorated bone healing and mineralization. Obvious observations are accelerated maturation of the new bone tissue, increased amount of bone deposition and a highly vascularized connective tissue. The lasered sockets showed massive infiltration of osteoblasts and large number of new blood vessels at 7 and 14 days post-extraction, indicating that diode laser stimulated osteoblast and endothelial cells differentiation. At 45 days post-extraction, the bone trabeculae of the lasered socket become more compact and assume a concentric lamellar pattern of Haversian system indicating mature bone formation. At the non-lasered sockets the bone was still immature with irregular bone trabeculae and large bone marrow spaces.
Our findings are in accordance with other studies. El-Maghraby [22] in a histological analysis showed a significant increase in the area of bone trabeculae, as well as the width of compact bone, for the lasered side of gamma irradiated mandibles of rats. Korany et al. [23] found that LLLT enhance bone repair in irradiated tooth sockets of albino rats. Garcia et al. [24] demonstrated that the LLLT was effective for stimulating bone formation in critical sized defects in the calvaria of rats submitted to ovariectomy. Nicola et al. [25] observed that applying LLLT on injured bones increased the bone volume, osteoblasts surface, and mineral apposition rates, suggesting that LLLT increased. Our results confirm the positive bio-stimulatory effect of LLLT, which depends mainly on the ability of the tissue to respond to light energy. The mechanisms underlying stimulation of bone healing may be multifactorial and include: promotion of angiogenesis [26], collagen production [27], osteogenic cell proliferation and differentiation [28]. Another study in the tibia of rats stated that the improvement of bone repair by LLLT was due to its role in upregulation of cyclooxygenase-2 expression in bone cells [29]. Current theories suggest that transcription of certain nuclear proteins, such as a rhodopsin-kinase enzyme may be photosensitive at certain wavelengths and this may be responsible for the accelerated wound healing capabilities of the LLLT [30].
Some studies focused on osteoblastic differentiation using LLLT. The results of three studies showed that LLLT was effective in the stimulation of osteoblasts differentiation [31–33]. On the other hand Bouvet-Gerbettaz et al. [34] and Coombe et al. [35] showed no beneficial effect of laser on the proliferation and differentiation of osteoblasts. The variation in the outcomes of LLLT application on bone repair may be attributed to different physical parameters used by different researchers. The main benefits of therapeutic laser seem to derive from the administering of doses, since there have been unfavorable results when the laser is used at higher levels or for extended periods of time [36]. However, Barbosa et al. [37], in evaluating the effect of different wavelength and time of application of LLLT in an experimental fracture model in rats confirmed that the positive effect of LLLT in bone repair is time- and wavelength-dependent. Wavelengths of diode lasers used in studies with positive results were 635, 685, 810, and 830 nm. Satisfactory results are obtained when the number of applications ranges from two to twelve low-energy sessions [38, 39]. The dose and frequency of laser application in our study lies within this range.
The beneficial effect of LLLT on socket healing in this study can be extrapolated to clinical situations, like treatment of alveolar osteitis after tooth extraction to hasten healing. The other possible clinical application of this study is the LLLT irradiation of dental implant bed to enhance osseointegration, especially in cases of immediate insertion and/or immediate loading.
Conclusion
Diode laser application significantly accelerated the healing of tooth extraction socket at early, as well as late stages of the healing process. The lasered sockets presented with greater number of mature bone trabeculae, dense collagen fibers and numerous blood vessels as compared to non-lasered sockets. The findings of the research strongly support the clinical application of low power laser therapy for treatment of delayed healing of tooth extraction socket, like alveolar osteitis.
Compliance with Ethical Standards
Conflict of interests
The authors declare that they have no conflict of interests. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
References
- 1.Hita-Iglesias P, Torres-Lagares D, Flores-Ruiz R, Magallanes-Abad N, Basallote-Gonzalez M, Gutierrez-Perez J. Effectiveness of chlorhexidine gel versus chlorhexidine rinse in reducing alveolar osteitis in mandibular third molar surgery. J Oral Maxillofac Surg. 2008;66(3):441–445. doi: 10.1016/j.joms.2007.06.641. [DOI] [PubMed] [Google Scholar]
- 2.Eshghpour M, Moradi A, Nejat AH. Dry socket following tooth extraction in an Iranian Dental Center: incidence and risk factors. J Dent Mater Tech. 2013;2(3):86–91. [Google Scholar]
- 3.Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9(1):87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 4.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37(4):411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 5.Kooistra BW, Jain A, Hanson BP. Electrical stimulation: nonunions. Indian J Orthop. 2009;43(2):149–155. doi: 10.4103/0019-5413.50849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adie S, Harris IA, Naylor JM, Rae H, Dao A, Yong S, Ying V. Pulsed electromagnetic field stimulation for acute tibial shaft fractures: a multicenter, double-blind, randomized trial. J Bone Joint Surg Am. 2011;93(17):1569–1576. doi: 10.2106/JBJS.J.00869. [DOI] [PubMed] [Google Scholar]
- 7.Hasuike A, Sato S, Udagawa A, Ando K, Arai Y, Ito K. In vivo bone regenerative effect of low-intensity pulsed ultrasound in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endodonto. 2011;111(1):12–20. doi: 10.1016/j.tripleo.2010.09.061. [DOI] [PubMed] [Google Scholar]
- 8.Fındık Y, Baykul T. Effects of low-intensity pulsed ultrasound on autogenous bone graft healing. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(3):255–260. doi: 10.1016/j.oooo.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 9.El-Ghannam A. Bone reconstruction: from bioceramics to tissue engineering. Expert Rev Med Devices. 2005;2(1):87–101. doi: 10.1586/17434440.2.1.87. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy TL, Ji C, Centrella M. Links among growth factors, hormones, and nuclear factors with essential roles in bone formation. Crit Rev Oral Biol Med. 2000;11(4):409–422. doi: 10.1177/10454411000110040201. [DOI] [PubMed] [Google Scholar]
- 11.Kazancioglu HO, Ezirganli S, Aydin MS. Effects of laser and ozone therapies on bone healing in the calvarial defects. Craniofac Surg. 2013;24(6):2141–2146. doi: 10.1097/SCS.0b013e3182a244ae. [DOI] [PubMed] [Google Scholar]
- 12.Nagata MJ, Santinoni CS, Pola NM, de Campos N, Messora MR, Bomfim SR, Ervolino E, Fucini SE, Faleiros PL, Garcia VG, Bosco AF. Bone marrow aspirate combined with low-level laser therapy: a new therapeutic approach to enhance bone healing. Photochem Photobiol B. 2013;121:6–14. doi: 10.1016/j.jphotobiol.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Walsh LJ. The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications. Aust Dent J. 1997;42(4):247–254. doi: 10.1111/j.1834-7819.1997.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki K, Shimizu N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med. 2000;26(3):282–291. doi: 10.1002/(sici)1096-9101(2000)26:3<282::aid-lsm6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Park JJ, Kang KL. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: a pilot study. Lasers Med Sci. 2012;27(1):223–230. doi: 10.1007/s10103-011-0944-8. [DOI] [PubMed] [Google Scholar]
- 16.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B Biol. 2005;81(2):98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Brawn PR, Kwong-Hing A. Histologic comparison of light emitting diode phototherapy-treated hydroxyapatite-grafted extraction sockets: a same-mouth case study. Implant Dent. 2007;16(2):204–211. doi: 10.1097/ID.0b013e318065a84c. [DOI] [PubMed] [Google Scholar]
- 18.Woodruff LD, Bounkeo JM, Brannon WM, Dawes KS, Barham CD, Waddell DL, Enwemeka S. The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Meta-analysis. Photomed Laser Surg. 2004;22(3):241–247. doi: 10.1089/1549541041438623. [DOI] [PubMed] [Google Scholar]
- 19.Nunamaker DM. Experimental models of fracture repair. Clin Orthop Relat Res. 1998;355:S56–S65. doi: 10.1097/00003086-199810001-00007. [DOI] [PubMed] [Google Scholar]
- 20.MacNeill SR, Cobb CM, Rapley JW, Glaros AG, Spencer P. In vivo comparison of synthetic osseous graft materials. A preliminary study. J Clin Periodontol. 1999;26(4):239–245. doi: 10.1034/j.1600-051x.1999.260407.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:298–307. [PubMed] [Google Scholar]
- 22.El-Maghraby EM, El-Rouby DH, Saafan AM. Assessment of the effect of low-energy diode laser irradiation on gamma irradiated rats’ mandibles. Arch Oral Biol. 2013;58(7):796–805. doi: 10.1016/j.archoralbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Korany NS, Mehannia SS, Hakam HM, El-Maghraby EMF. Evaluation of socket healing in irradiated rats after diode laser exposure (histological and morphometric studies) Arch Oral Biol. 2012;57(7):884–891. doi: 10.1016/j.archoralbio.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Garcia VG, da Conceição JM, Fernandes LA, de Almeida JM, Nagata MJ, Bosco AF, Theodoro LH. Effects of LLLT in combination with bisphosphonate on bone healing in critical size defects: a histological and histometric study in rat calvaria. Lasers Med Sci. 2013;28(2):407–414. doi: 10.1007/s10103-012-1068-5. [DOI] [PubMed] [Google Scholar]
- 25.Nicola RA, Jorgetti Y, Rigau J, Pacheco MT, dos Reis LM, Zangaro RA. Effect of low-power GaAlAs laser (660 nm) on bone structure and cell activity: an experimental animal study. Lasers Med Sci. 2003;18(2):89–94. doi: 10.1007/s10103-003-0260-z. [DOI] [PubMed] [Google Scholar]
- 26.Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O, Wolf T, Ben Haim S. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers Surg Med. 2001;28(3):204–211. doi: 10.1002/lsm.1039. [DOI] [PubMed] [Google Scholar]
- 27.Ayuk SM, Houreld NN, Abrahamse H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol Ther. 2012;14(12):1110–1117. doi: 10.1089/dia.2012.0125. [DOI] [PubMed] [Google Scholar]
- 28.Yaakobi T, Maltz L, Uoron U. Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He–Ne) irradiation. Calcif Tissue Int. 1996;59(4):297–300. doi: 10.1007/s002239900126. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro DA, Matsumoto MA. Low-level laser therapy improves bone repair in rats treated with anti-inflammatory drugs. J Oral Rehabil. 2008;35(12):925–933. doi: 10.1111/j.1365-2842.2008.01891.x. [DOI] [PubMed] [Google Scholar]
- 30.Bliziotes M, Murtagh J, Wiren K. Beta-adrenergic receptor kinase-like activity and beta-arrestin are expressed in osteoblastic cells. J Bone Miner Res. 1996;11(6):820–826. doi: 10.1002/jbmr.5650110613. [DOI] [PubMed] [Google Scholar]
- 31.Petri AD, Teixeira LN, Crippa GE, Beloti MM, de Oliveira PT, Rosa AL. Effects of low level laser therapy on human osteoblastic cells grown on titanium. Braz Dent J. 2010;21(6):491–498. doi: 10.1590/s0103-64402010000600003. [DOI] [PubMed] [Google Scholar]
- 32.Soleimani M, Abbasnia E, Fathi M, Sahraei H, Fathi Y, Kaka G. The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts: an in vitro study. Lasers Med Sci. 2012;27(2):423–430. doi: 10.1007/s10103-011-0930-1. [DOI] [PubMed] [Google Scholar]
- 33.Saygun I, Nizam N, Ural AU, Serdar MA, Avcu F, Tozum TF. Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Photomed Laser Surg. 2012;30(3):149–154. doi: 10.1089/pho.2011.3079. [DOI] [PubMed] [Google Scholar]
- 34.Bouvet-Gerbettaz S, Merigo E, Rocca JP, Carle GF, Rochet N. Effects of low-level laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoclasts. Lasers Surg Med. 2009;41(4):291–297. doi: 10.1002/lsm.20759. [DOI] [PubMed] [Google Scholar]
- 35.Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res. 2001;4(1):3–14. doi: 10.1034/j.1600-0544.2001.040102.x. [DOI] [PubMed] [Google Scholar]
- 36.Gordjestani M, Dermaut L, Thierens H. Infrared laser and bone metabolism: a pilot study. Int J Oral Maxillofac Surg. 1994;23(1):54–56. doi: 10.1016/s0901-5027(05)80329-7. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa D, de Souza RA, Xavier M, da Silva FF, Arisawa EA, Villaverde AG. Effects of low-level laser therapy (LLLT) on bone repair in rats: optical densitometry analysis. Lasers Med Sci. 2013;28(2):651–656. doi: 10.1007/s10103-012-1125-0. [DOI] [PubMed] [Google Scholar]
- 38.Guzzardella GA, Torricelli P, Nicoli Aldini N, Giardino R. Laser technology in orthopedics: preliminary study on low power laser therapy to improve the bone-biomaterial interface. Int J Artif Organs. 2001;24(12):898–902. [PubMed] [Google Scholar]
- 39.Guzzardella GA, Fini M, Torricelli P, Giavaresi G, Giardino R. Laser stimulation on bone defect healing: an in vitro study. Laser Med Sci. 2002;17(3):216–220. doi: 10.1007/s101030200031. [DOI] [PubMed] [Google Scholar]