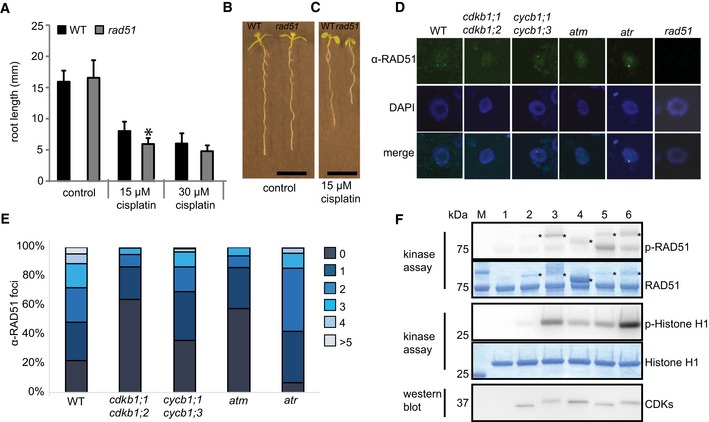
WT and rad51 mutants were grown on control plates or transferred to plates supplemented with 15 or 30 μM cisplatin, respectively, 3 days after germination. Root lengths were measured 3 days after transfer and the net root growth of 3 days is shown in the graph. Asterisk indicates significant differences within a 5% confidence interval (Student's t‐test). Three biological replicates, each containing at least 15 plants, were analyzed. The mean of the root length of each individual experiment was determined and again averaged for the three biological replicates. Graphs represent mean ± SD.
Image shows a wild‐type plant (left) and rad51 mutant (right) grown on control plates. Images were taken 6 days after germination. Scale bar: 1 cm.
Image shows a wild‐type plant (left) and rad51 mutant (right) germinated on control plates and transferred to plates supplemented with 15 μM cisplatin 3 days after germination. Images were taken 6 days after germination, that is, 3 days after transfer to cisplatin. Scale bar: 1 cm.
Immunostaining of RAD51 foci in the wild‐type and indicated mutant cells after 2 h of treatment with 50 μM cisplatin.
Counted numbers of RAD51 foci per cell detected after 2 h of treatment with 50 μM cisplatin in wild‐type and mutant plants. For each sample, the RAD51 foci of 100 cells were counted and grouped into six categories: cells with 0, 1, 2, 3, 4, or > 5 foci per cell.
In vitro kinase assay of purified CDK complexes phosphorylating RAD51. RAD51 and histone H1 kinase assays were performed with [γ‐32P]ATP as a phosphate donor. Proteins were subjected to SDS–PAGE after the kinase reaction and stained with Coomassie Brilliant Blue demonstrating the equal loading of the substrates (lower panels). Phosphorylated proteins were detected by autoradiography (upper panels). The reactions were normalized by using equal amounts of CDKs assuring equal levels of active CDK‐cyclin complexes; the protein blot indicates the relative amounts of the CDKs in the reaction (bottom panel). Abbreviation: p‐RAD51 and p‐histone H1 for [32P]‐phosphorylated MBP‐RAD51‐His6 and recombinant human histone H1, respectively, resulting from kinase assays with radiolabeled ATP. Asterisks indicate varying amounts of cyclins that can be in the reaction due to purification procedure. 1: without kinase, 2: CDKA;1‐CYCA2;3, 3: CDKB1;1‐CYCA2;3, 4: CDKB1;1‐CYCD2;1, 5: CDKA;1‐CYCB1;1, 6: CDKB1;1‐CYCB1;1.