Figure EV6. Purification of Hrr25 and Hrr25:Mam1 for ATPase assays and modeling of Mam1 Arg131 interaction with nucleotide and CK1‐7.
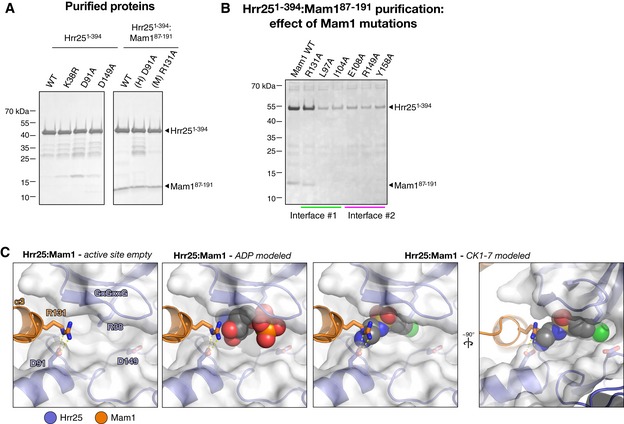
- SDS–PAGE analysis of purified Saccharomyces cerevisiae Hrr251–394 and Hrr251–394:Mam187–191 complexes used for ATPase assays.
- Ni2+ affinity pull‐down of co‐expressed His6‐Hrr251–394 K38R:Mam187–191 complexes with mutations to Mam1 in interfaces #1 or #2. Except for R131A, all mutations result in a loss of co‐purification of Mam1, indicating a loss of binding, and a significant reduction in the level of soluble Hrr251–394 K38R.
- Close‐up views of the Hrr25 active site in the structure of S. cerevisiae Hrr251–394:Mam187–191. Mam1 R131 extends toward the ATP‐binding pocket, and while it would not clash with a bound nucleotide (center panel), it would clash with the aminoethyl group of bound CK1‐7 (right panels).
