Figure 8. Hrr25 phosphorylates Dsn1.
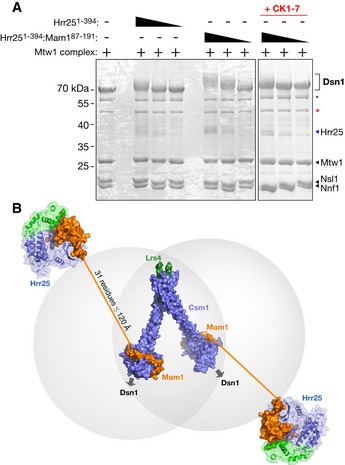
- Phos‐tag gels showing phosphorylation of purified S. cerevisiae Mtw1 complex by Hrr251–394 and Hrr251–394:Mam187–191. Asterisks denote N‐terminal proteolytic cleavage products of Dsn1, with the red asterisk denoting a previously characterized product lacking the first 171 residues (Hornung et al, 2011).
- Model of the intact monopolin complex. Four copies of Csm1 (blue) and two of Lrs4 (green) form the core “V”‐shaped kinetochore‐binding complex (Corbett et al, 2010). Each dimer of Csm1 binds one Mam1 (orange) (Corbett & Harrison, 2012), which in turn recruits one copy of Hrr25 (blue/green). Thirty‐one residues of Mam1 (192–222) separate the protein's Hrr25‐binding and Csm1‐binding domains, which when fully extended could reach as far as ˜120 Å.
