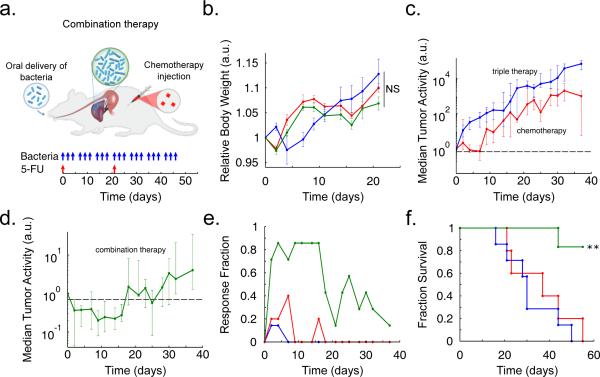
Fig. 5.
In vivo testing in an experimental model of colorectal metastases in the liver via oral delivery of bacteria. (a) Schematic of the experimental syngeneic transplantation model of hepatic colorectal metastases in a mouse, with the dosing schedule of either engineered bacteria (SLC-3) or a common cytotoxic chemotherapeutic, the antimetabolite 5-FU. The SLC-3 strains were delivered orally while 5-FU was delivered via intraperitoneal injection. (b) Relative body weight over time for the mice with with hepatic colorectal metastases fed with the SLC-3 strains (blue line), injected with 5-FU chemotherapy (red line), or a combination of the two (green line). Error bars indicate ±1 standard error for 5 - 7 mice. (c) Median relative tumor activity, measured via tumor cell luminescence using IVIS imaging, for the chemotherapy and SLC-3 cases from (b). (d) Median relative tumor activity for the combination therapy case from (b). Error bars for (c) and (d) indicate the interquartile ranges for 5 - 7 mice. The dashed line marks relative tumor activity of 0.70. (e) Fraction of mice from the cases in (b) which respond with 30% reduction of tumor activity over time. (f) Fraction survival over time for the mice in (b) (**P < 0.01, log rank test; n = 5 - 7 mice).