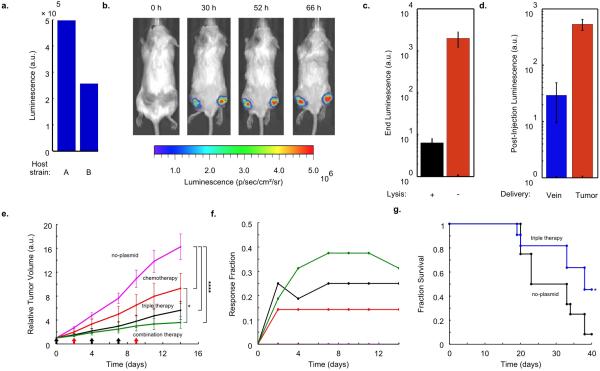
Extended Data Fig. 3.
In vivo expression and therapy testing. (a) End-point in vitro luminescence intensity for SLC strains after ~20 h of growth. Host strains A and B are the host bacteria for Strains 8 and 10. They are ELH1301 and ELH 430, respectively. Host A exhibits ~2-fold higher luminescence with the same circuit than Host B. (b) IVIS imaging over time of a mouse bearing subcutaneous tumors injected with a genomically integrated constitutively luminescent strain (Strain 9). (c) End-point in vivo bacterial luminescence of the SLC-hly strain and the constitutively luminescent strain from the experiments presented in Fig. 4. Error bars represent the standard error of the mean bacterial luminescence from 9 tumors. (d) Post-injection in vivo bacterial luminescence for the constitutively luminescent strain administered intravenously (vein) or intratumorally (tumor). Luminescence was measured ~20 h post-injection. Error bars represent the standard error of the mean bacterial luminescence from 6 and 9 tumors for the intravenous and intratumoral cases, respectively. (e) Average relative tumor volume over time for subcutaneous tumor bearing mice injected with the no-plasmid bacterium (Strain 7), 5-FU chemotherapy, the SLC-3 strains, and the combination of SLC-3 with chemotherapy. Bacteria were injected intratu-morally on days 0, 4, and 7 (black arrows), and chemotherapy was administered on days 2 and 9 (red arrows) (*P < 0.05, ****P < 0.0001, two-way ANOVA with Bonferroni post test, n = 12 - 16 tumors, s.e.). (f) Fraction of mice from the cases in (e) which respond with 30% reduction of tumor volume over time. (g) Fraction survival over time for mice with hepatic colorectal metastases fed with either the SLC-3 strains (blue line) or the no-plasmid control (black line) (*P < 0.05, log rank test; n = 11 - 12 mice).