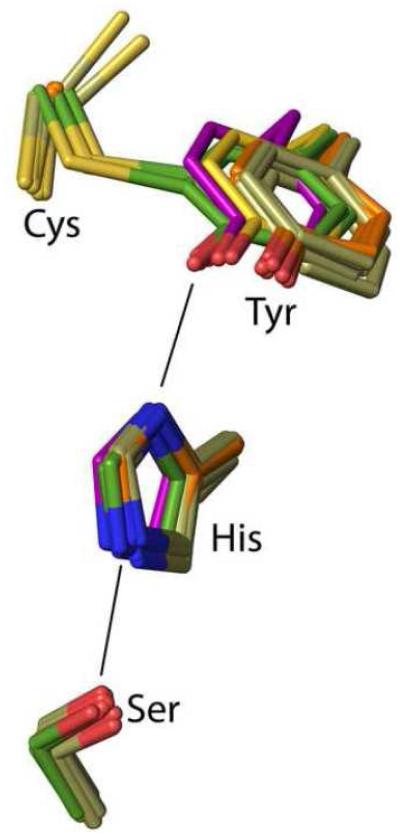
Figure 8.
Positional conservation of the Ser-His-Tyr catalytic triad among wild-type CDO structures. Side chains equivalent to rat CDO residues 93, 153, 155 and 157 are shown for wild-type rat CDO structures (green carbons; six inhibitor-bound structures from this work plus the previously published unliganded–PDB code 2B5H20 – and Cys-persulfenate-bound – PDB code 4IEU28 – structures), its C93A variant (orange carbons; 6 structures from this work), its Y157F variant (tan carbons; 10 structures from this work), along with previously published structures of its C93G variant (cream carbons; PDB code 4UBG37) and the non-crosslinked, but fully active Bacillus subtilis CDO (purple carbons; PDB code 4QM936).