Fig. 1.
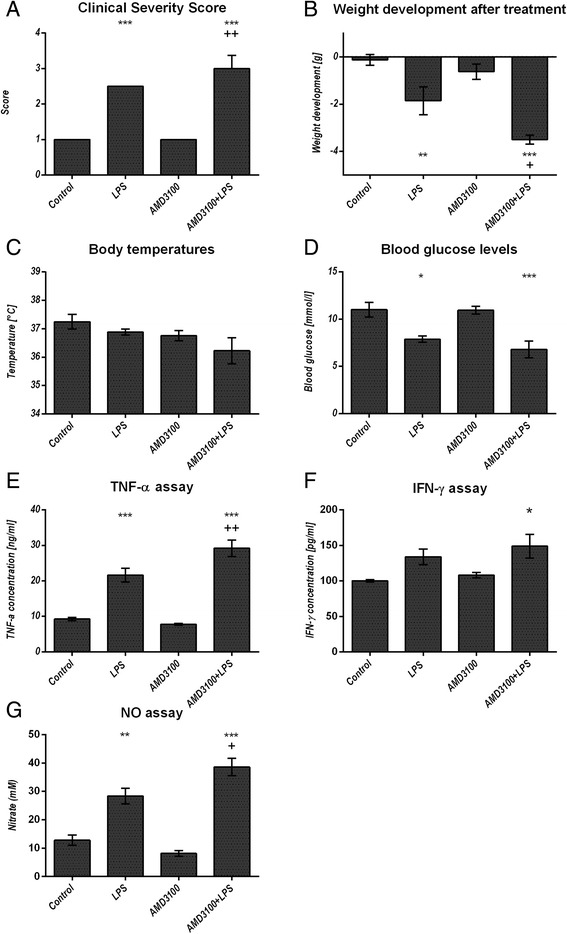
General condition and systemic parameters. C57BL/6 N mice were treated either with LPS (5 mg/kg body weight), AMD3100 (5 mg/kg body weight), with both substances or with the solvent PBS (control). 24 h after LPS administration, the Clinical Severity Score (a), the body weight (b) and the body temperature (c) were assessed and the mice were sacrificed. Blood glucose content was determined from whole blood (d) and serum was obtained for TNF alpha, interferon (IFN) gamma and nitric oxide (NO) measurements (e-g). Data are given as mean ± standard error of the mean (SEM) or as median with interquartile ranges (CSS), respectively; n = 7 for each group. Statistical significant differences between the different treatment groups were determined by using the one-way analysis of variance (ANOVA) and the Tukey post hoc test, except for the CSS, which was analyzed by the non-parametric Kruskal-Wallis test followed by the Mann-Whitney-U test. They are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. control animals; +, p < 0.05; ++, p < 0.01; +++, p < 0.001 vs. LPS treatment
