Figure 3.
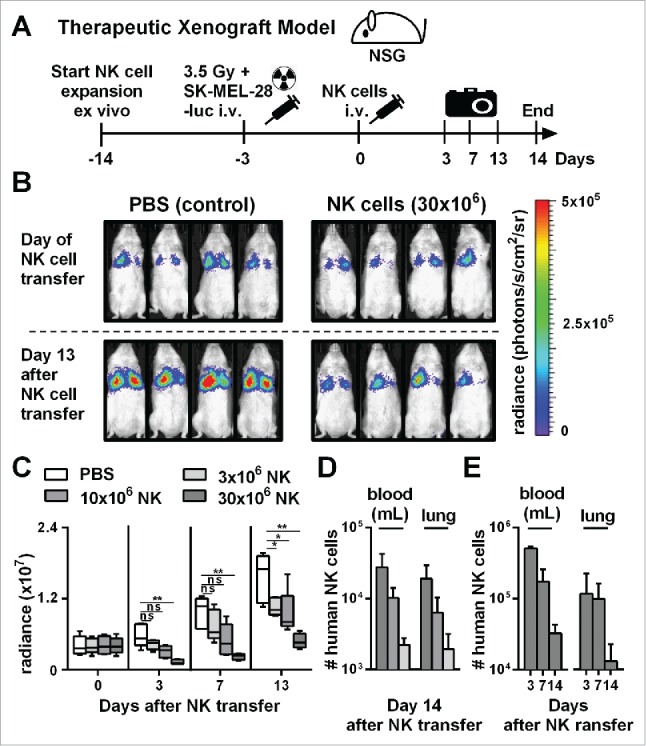
Adoptive transfer of NK cells expanded by the optimized protocol in tumor-bearing mice resulted in pronounced tumor growth control. (A) Scheme is shown for evaluation of expanded NK cells in vivo using a xenograft model. Mice were irradiated and received human SK-MEL-28 melanoma cells-expressing luciferase by intravenous (i.v.) injection. Three days later, after tumor engraftment, the mice were treated (i.v.) with human NK cells expanded with the optimized protocol and the tumor load was monitored by luciferase activity. IL-2 was repeatedly injected intraperitoneally. (B) Tumor-bearing mice, as described in A, were treated with PBS, as a control, or with 30 × 106 NK cells that were expanded for 14 d using the optimized expansion protocol. The pictures display the bioluminescence (radiance) showing the in vivo luciferase activity of four representative mice of each group at the day of NK cell transfer (top) and 13 d thereafter (bottom). (C) Tumor-bearing mice were treated with NK cells as described in B using different NK cell doses. Mean and range of the tumor burden, measured by bioluminescence, is shown at different time points for one representative experiment with 4–5 mice per group. (D) Mice were treated as described in C, and the transferred human NK cells were re-isolated from blood and lungs of the mice 14 d after NK cell injection and were enumerated using flow cytometry. Mean and standard deviation of NK cell numbers per lung and per mL of blood are shown for four mice per group. (E) Tumor-bearing mice were treated with 30 × 106 expanded NK cells as described in B and at day 3, 7, and 14 the mice were sacrificed and human NK cells were re-isolated from blood and lungs. Mean and standard deviation of NK cell numbers per lung or mL of blood are shown for four mice per group. Statistical significance in all experiments was tested by Student's t-test.
