Figure 3.
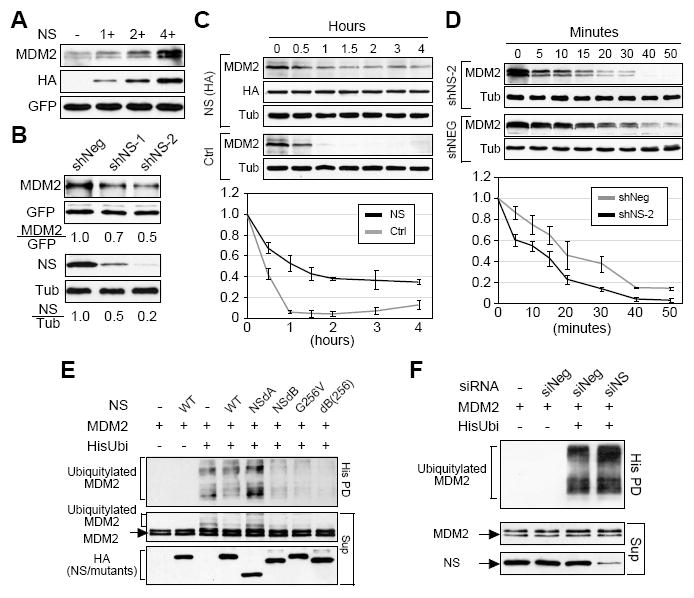
NS increases the protein level of MDM2 by preventing its degradation and ubiquitylation.
(A) Coexpression of NS (HA-tagged) increased the protein levels of exogenously expressed MDM2 in a dose-dependent manner. (B) Knocking down the expression of NS by the NS-targeting shRNAmir constructs (shNS-1 and shNS-2) decreased the amount of MDM2 protein. (C) MDM2 protein stability, with or without NS overexpression (NS v.s. Ctrl), was measured in H1299 cells. After cycloheximide treatment, cell lysates were collected from 0 to 4 hours (h) at 0.5-1h intervals. The MDM2 protein amounts at every time point were measured from three independent experiments, adjusted based on their α-tubulin amounts, and expressed as percentages of the MDM2 protein amount at the 0h time-point. (D) NS depletion by shNS-2 cotransfection increased the protein degradation of MDM2. Protein degradation assays were performed over a 50-minute (m) window. (E) HEK293 cells were transfected with (His)6-tagged ubiquitin, MDM2, and/or NS (wild-type or mutant) plasmids as specified. Ubiquitylated MDM2 products were pulled down by Ni2+ sepharose (His PD) and detected by anti-MDM2 (SMP14) antibody. Overexpression of wild-type NS slightly decreased the polyubiquitylation of MDM2 compared to the control sample. Overexpression of the nucleoplasmic mutants of NS (dB, G256V, and dB(256)) significantly decreased the ubiquitylated products of MDM2. Sup, supernatant. (F) Conversely, depleting the endogenous NS by the NS-targeting siRNA (siNS) increased the ubiquitylation of MDM2 compared to cells treated with a scrambled siRNA (siScr).
