Figure 4.
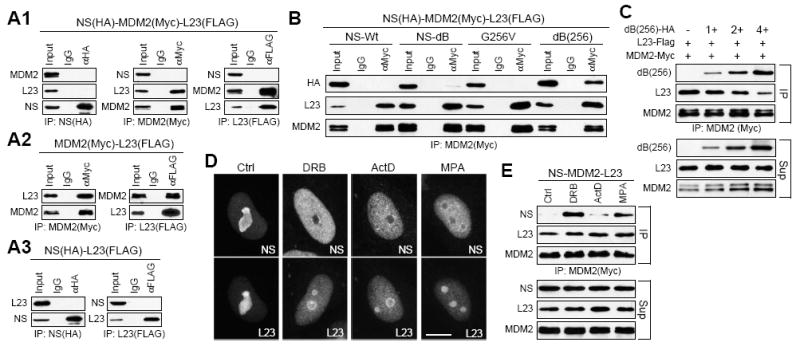
Nucleoplasmic NS competes against L23 for MDM2 binding.
(A1) Triple coIP of NS (HA), MDM2 (Myc), and L23 (FLAG) by the indicated antibodies (IP) showed that L23 coexpression reduces the interaction between NS and MDM2. Double coIP showed that L23 interacts with MDM2 (A2) but not with NS (A3). (B) The ability of NS to bind MDM2 in the presence of L23 overexpression was significantly increased by the combined mutations of dB and G256V (dB(256)). (C) The dB(256) mutant was capable of competing against L23 for MDM2 binding in the triple-coIP experiments. The MDM2 protein complexes (top panel) were immunoprecipitated from lysates (bottom panel) containing the same amount of L23 and increasing amounts of dB(256). (D) Both NS (HA) and L23 (FLAG) reside primarily in the nucleolus under normal growth conditions (Ctrl). When exposed to ADR (2uM), actinomycin-D (ActD, 0.05ug/ml) and MPA (40uM) for 4h, L23 was redistributed to the nucleoplasm as NS but to a less extent. Distributions of NS and L23 were shown in different ADR-treated cells due to the autofluorescent property of ADR. Scale bar shows 10um. (E) Compared to mock-treated cells, ADR and MPA significantly increased the binding between NS and MDM2, and ActD had a smaller effect. (F) Proteins were extracted from cells coexpressing HA-tagged NS, Myc-tagged MDM2, and FLAG-tagged L5, L11, or L23, and immunoprecipitated by anti-Myc antibody. Western blots showed that L23 competes against NS for MDM2 binding better than L5 and L11 do.
