ABSTRACT
We here show that living in a stimulus‐rich environment (ENR) improves water maze learning with respect to specific key indicators that in previous loss‐of‐function experiments have been shown to rely on adult hippocampal neurogenesis. Analyzing the strategies employed by mice to locate the hidden platform in the water maze revealed that ENR facilitated task acquisition by increasing the probability to use effective search strategies. ENR also enhanced the animals’ behavioral flexibility, when the escape platform was moved to a new location. Treatment with temozolomide, which is known to reduce adult neurogenesis, abolished the effects of ENR on both acquisition and flexibility, while leaving other aspects of water maze learning untouched. These characteristic effects and interdependencies were not seen in parallel experiments with voluntary wheel running (RUN), a second pro‐neurogenic behavioral stimulus. Since the histological assessment of adult neurogenesis is by necessity an end‐point measure, the levels of neurogenesis over the course of the experiment can only be inferred and the present study focused on behavioral parameters as analytical endpoints. Although the correlation of physical activity with precursor cell proliferation and of learning and the survival of new neurons is well established, how the specific functional effects described here relate to dynamic changes in the stem cell niche remains to be addressed. Nevertheless, our findings support the hypothesis that adult neurogenesis is a critical mechanism underlying the beneficial effects of leading an active live, rich in experiences. © 2015 The Authors Hippocampus Published by Wiley Periodicals, Inc.
Keywords: hippocampus, plasticity, stem cells, memory, environmental enrichment, exercise
Abbreviations used
- ENR
mice were housed under enriched environment conditions
- RUN
mice had access to voluntary wheel running
- TMZ
mice were treated with cytostatic drug temozolomide.
INTRODUCTION
Adult hippocampal neurogenesis is a particular type of structural plasticity that is regulated by behavioral activity and presumably improves hippocampal function (Kempermann, 2012). What exactly this functional contribution is has been a matter of debate, although an increasingly clearer picture emerges (Aimone et al., 2009; Clelland et al., 2009; Deng et al., 2010; Appleby et al., 2011; Sahay et al., 2011). An interesting twist to this is the idea that this kind of plasticity might actually be related to “forgetting,” which can be interpreted as a particular type of dealing with memories (Akers et al., 2014). Our hypothesis is that new neurons add flexibility to hippocampus‐dependent learning in situations, in which novel bits of information have to be integrated into previously established contexts (Appleby et al., 2011; Garthe and Kempermann, 2013).
Studies by us and others suggest that in the spatial navigation task of the Morris water maze, as a gold standard test of hippocampal function in rodents, a reduction of adult hippocampal neurogenesis results in specific, albeit often subtle impairments (Snyder et al., 2005; Winocur et al., 2006; Zhang et al., 2008; Dupret et al., 2008; Jessberger et al., 2009; Garthe et al., 2009, 2014; Martinez‐Canabal et al., 2013). We have argued that the specific task design must be such that it is sensitive to exactly those functional aspects attributable to adult neurogenesis in order to find the effect (Garthe and Kempermann, 2013). Since the presumed function of adult neurogenesis is to optimize an already existing network in the hippocampus, the complexity and degree of difficulty of a given experimental setup affects the extent to which the new neurons are getting involved. Other water maze setups and protocols might emphasize other aspects and miss these contributions.
The urgent question now is, whether the plastic interaction between behavior and neurogenesis is indeed reciprocal. While there has been interesting circumstantial and direct evidence that increasing adult neurogenesis is associated with improvements in learning and memory, those experiments were done using highly reductionistic tasks or in models, in which the increase in neurogenesis was due to a nonphysiological intervention. The question thus arose, whether an increase in neurogenesis due to an animal's behavior would be followed by behavioral changes and improvements that can be directly linked to adult hippocampal neurogenesis and its regulation.
This hypothesis is in line with our previous finding that stable individual behavioral trajectories that develop in genetically identical animals sharing one large enclosure explain 22% of the variance found in adult neurogenesis (Freund et al., 2013). The measured behaviors in that study were related to motility. Physical activity (RUN) is a strong inducer of adult hippocampal neurogenesis and we have now found that normal locomotion in the cage, in the absence of a running wheel also correlates with adult neurogenesis.
Intriguingly, however, plain locomotion explained less variance than the particular parameter analyzed in that study, called roaming entropy, a measure of territorial coverage. Roaming entropy supposedly includes qualitative aspects of exploration and experience and thus relates to the cognitive challenges posed by living in a stimulus‐rich environment. If in an even more reductionist design, a period of access to a running wheel was followed by exposure to an enriched environment (ENR) the individual pro‐neurogenic effects of the two types of intervention (nonspecific, physical vs. specific, cognitive) become additive, further underscoring the hypothesized physiological link between locomotion and cognition.
In the present study we again applied the two individual pro‐neurogenic stimuli RUN and ENR but in half of the groups combined them with a treatment with temozolomide (TMZ), a cytostatic drug that can be used with advantage to experimentally suppress adult neurogenesis (Garthe et al., 2009). We assessed water maze performance in these mice and focused on specifically those aspects that in our previous study were found to be associated with adult neurogenesis. We asked for the contribution of adult neurogenesis to improvements in learning performance after exposure to RUN and ENR. Note, that the particular design of the study does not allow to measure adult neurogenesis at the time‐point of training or testing, because adult neurogenesis as assessed by the classical BrdU‐method can only be quantified postmortem. That, in general, RUN and ENR increase adult neurogenesis (Kempermann et al., 1997; Van Praag et al., 1999; Kronenberg et al., 2003; Fabel et al., 2009) and TMZ suppresses it (Garthe et al., 2009; Niibori et al., 2012; Nokia et al., 2012), has been firmly established in numerous studies.
MATERIAL AND METHODS
Animals and Tissue Preparation
For all experiments we used 6‐ to 8‐week old female C57BL/6N mice (Charles River), 10 per group. The mice were kept at the animal facility of the Medical Faculty of Technische Universität Dresden, Germany. All applicable local and federal regulations of animal welfare in research were followed. The experiments were approved by the responsible authority, Landesdirektion Dresden.
Drug Treatment
Temozolomide (TMZ, Temodal® or Temodar®, SP Europe, Belgium) is an alkylating agent used for the treatment of recurrent malignant glioma. To suppress adult neurogenesis, mice from the treatment groups (TMZ) received injections of TMZ at 50 mg/kg (i.p., 2.5 mg/ml in 0.9% NaCl), whereas the control group (CTR) received sham injections of saline only. This regimen was given on the first 3 days of a week for 4 weeks to match protocols used for glioma treatment in humans.
Behavioral testing started 3 weeks after the final TMZ injection. Thus, when the mice were learning the water maze task, the number of new granule cells younger than 7 to 8 weeks of age was substantially reduced. Adult‐born granule cells become recruitable at ∼28 days of age and were shown to be selected during acquisition of a spatial learning task at 6 to 8 weeks (Kee et al., 2007; Tashiro et al., 2007). Suppressing proliferation for 4 weeks combined with a recovery period of 3 more weeks ensured that most of the proliferating cells born before onset of TMZ treatment would already have been used or eliminated. At the time‐point of the behavioral analysis the subpopulation of 4‐ to 8‐week old adult generated neurons is primarily affected by TMZ. For a detailed analysis of the TMZ effects on adult neurogenesis and the results of numerous control experiments to rule out non‐specific effects see Garthe et al. (2009).
Morris Water Maze Task
Three weeks after the end of TMZ treatment, mice were trained in the reference memory version of the Morris water maze task to locate a hidden escape platform in a circular pool (2 m diameter). Water was made opaque with non‐toxic titan dioxide and kept at a temperature of 19 to 20°C. Each mouse was given six trials a day for 5 consecutive days in the first testing week (acquisition 1), followed by two consecutive days in the following week (acquisitions 2 and 3).
After this, the experimental design contained two additional acquisition and reversal periods after a 4‐week break under standard housing conditions, which were not analyzed for the present study.
The intertrial interval was 30 min. The position of the platform was changed after day 3 to the opposite quadrant (Fig. 1a). Mice were released from one of four possible starting points and allowed to search for the hidden platform for a maximum of 120 s. During each day the starting position remained constant. At the end of each trial and irrespective of trial performance, mice were guided to the platform and allowed to remain there for at least 15 s. Swim paths were recorded using Ethovision (Noldus) and further analyzed using Matlab (The Mathworks).
Figure 1.
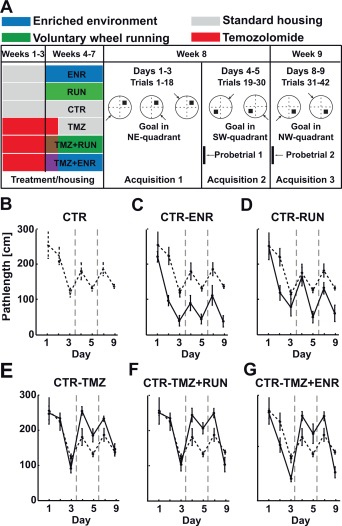
Experimental protocol and swim path lengths. (a) The water maze protocol consisted of multiple acquisitions followed by goal reversals. The first 30s of the first trial after each reversal were considered as probe trials. The original experimental design also included a second period of behavioral testing (shaded), which was not subject of the analysis in the present study. (b–g) Path length to reach the hidden platform. Shown are means ± standard deviations. Control values are always connected by dashed lines. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Running Wheel Assay and Enriched Environment Design
Mice from the RUN group had access to running wheels in their home cages throughout light and dark periods and were allowed to use them freely. For the ENR group mice were housed in a single larger group of 10 animals (as opposed to five per cage under standard conditions) allowing more differentiated social interactions. ENR mice lived in a larger cage (0.8 × 0.8 m) equipped with toys, houses, and a maze‐like tube system that changed its configuration every week providing numerous opportunities for learning.
Morris Water Maze Data Analysis
General spatial learning was analyzed using classical parameters like latency to reach the platform, swim path length, and relative time spent in the four quadrants (Figs. 1 and 2).
Figure 2.
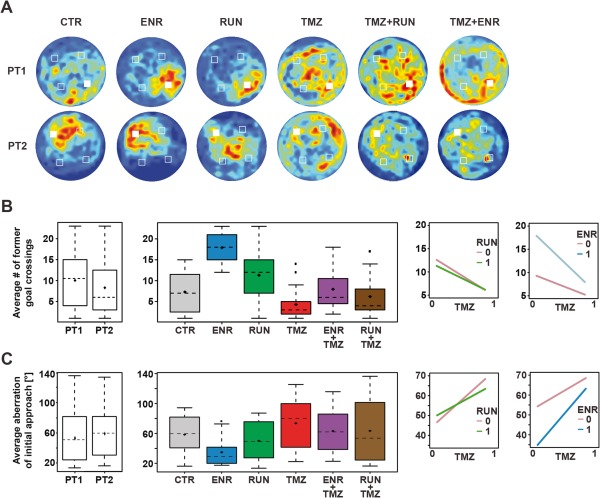
Probe trial performance. (a) Probe trial performance indicated by heat maps. Dark‐red zones represent a sixfold presence probability. (b) Probe trial performance indicated by number of crossings of former goal positions. From left to right: comparison of the distribution of former goal crossings for probe trials 1 and 2; distributions of goal crossings in the experimental groups (pooled over both probe trials for reasons of simplicity. Since we found a significant effect of probe trial (P = 0.034) it was included in all ANOVA models used to analyze the effects of ENR, RUN, and TMZ); interaction plots illustrating the effect of TMZ on the different levels of RUN/ENR by connecting the corresponding mean values with straight lines. Boxplots are showing the median (dashed horizontal line), the interquartile range (whiskers), the mean (diamonds), and potential outliers (squares). (c) Average directional aberration of initial goal approach; see panel b) for description of plot parameters. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To visualize spatial preference for the goal position in heat map‐like occupancy plots the MWM pool was divided into 10 × 10 cm wide sectors and the probability calculated of an animal to be found in each sector.
Aberration of initial heading direction was calculated as previously described in Woolley et al. (2010).
For analysis of search strategies swim path data from Ethovision (Noldus, NL) were used to derive the time‐tagged x,y‐coordinates for classifying the respective predominant search strategies by an algorithm implemented in Matlab (Garthe et al., 2009 for details). Classification criteria served an only descriptive function and are not meant to prove the involvement of particular cognitive processes or brain structures.
Statistics
For testing the effects of ENR, RUN, and TMZ as well as of acquisition phase and time (if appropriate) on path length, swim speed, average aberration, number of goal crossings, and trials to criterion, we applied analysis of variance (ANOVA) models. Within this framework testing of main and interaction effects was done using F‐test statistics. Because the identity of individual mice could not be traced back, we averaged daily measurements (six trials, 10 mice) of path length and swim speed before calculating ANOVA models to avoid inflated error rates according to pseudo‐replicates.
For statistical analysis of the effect of ENR, RUN, and TMZ on the search strategy, we applied a logistic regression (LR). Using the LR model we estimated the odds ratios for the application of ENR, RUN, and TMZ. That means, we compared the chance of using more versus less hippocampus‐dependent search strategies between the above listed situations and the control setting, which does neither apply ENR, RUN, nor TMZ. Corresponding P values are based on the Wald‐test statistics.
Parameters of the ANOVA and the LR models where estimated by a maximum likelihood approach. The minimal adequate model was determined by a stepwise comparison of models using likelihood‐ratio tests. Appropriateness of ANOVA/LR models was checked by analyzing the residuals. All calculations were done using the statistical programming environment R.
RESULTS
Performance in Water Maze Learning is Affected by Exposure to a Stimulus‐Enriched Environment and Suppression of Adult Hippocampal Neurogenesis
We first asked whether exposure to RUN or ENR would affect spatial learning in the water maze task as measured by general parameters like overall learning performance, spatially specific memory for learned goal locations, and the directional acuity of routes selected for initial goal approaches. Compared with controls, ENR animals showed shorter swim path lengths to find the hidden platform (P < 0.001, Fig. 1c). For both probe trials, ENR also resulted in a significantly greater number of crossings of the former goal location (P < 0.001, Figs. 2a–c), indicating a strong and specific memory of that position. Because we found an effect of probe trial on the number of former goal crossings (P = 0.034), it was included in the statistical model used for ANOVA to analyze the effects of ENR, RUN and TMZ (Fig. 2b; for reasons of simplicity, the figure displays the pooled data from both probe trials).
We also analyzed the average directional aberration of initial approaches towards the expected platform, a parameter introduced by Woolley et al. (2010). In contrast to an average heading direction (a commonly assessed parameter in the water maze), the initial heading direction (or initial heading error) only considers the swim path until the cumulative distance had equaled the direct start‐goal connection. Good learners possessing a full and specific cognitive map show initial goal approaches that bring the animals close to the hidden platform and thus show a small initial heading error. In line with the superior results regarding path length, mice exposed to ENR showed a significantly smaller directional aberration for initial approaches towards the goal (P = 0.0117, Fig. 2c).
Although mice with access to running wheels (RUN) showed a robust trend towards shorter path length (P = 0.0512), they neither showed significantly more former goal crossings (P = 0.4807) nor in their directional aberration of the first approach (P = 0.891; Figs. 1d and 2b,c).
In contrast, mice with a substantial reduction in adult neurogenesis (after TMZ treatment) showed a lower number of former goal crossings (P < 0.001, Figs. 2a,b) and a larger directional aberration of their initial approaches during probe trials (P < 0.001, Fig. 2c). Although there was a significant global increase in path length (TMZ‐effect: P < 0.001; Fig. 1e), treated mice generally learned to navigate to the hidden platform as indicated by decreasing path lengths within acquisitions (comparison of averaged path length for days 1 and 3: P < 0.001), even though they used less effective search trajectories. For further characterization of TMZ‐treated mice, specifically to rule out nonspecific effects of the treatment, see the extensive set of experiments in Garthe et al. (2009).
For the number of former goal crossings the multivariate statistical analyses revealed a highly significant interaction of TMZ‐treatment with living under ENR conditions (P < 0.001, Fig. 2b), suggesting that the positive effect of ENR on the number of former goal crossings to a significant degree relies on the presence of new hippocampal neurons. However, for path length and aberration of initial approaches no significant interaction effects were found (Figs. 1b–g and 2c). For all experimental groups, we found a strong day‐within‐acquisition effect (P < 0.001), indicating that all mice irrespective of the housing and treatment conditions successfully learned to find the hidden platform.
Taken together, for specific parameters spatial learning in the water maze appears to be affected by ENR and TMZ in a complementary manner. Living in a stimulus‐rich environment, which is known to increase the number of new neurons, improved spatial learning, whereas suppression of adult neurogenesis impaired water maze performance. How reliable and precise mice crossed the former learned goal position was found to be affected by both ENR and TMZ, whose shared feature is the presence or absence of new hippocampal neurons.
Although ENR, RUN, and TMZ all showed a significant effect on swim speed (P < 0.001 for all variables, Supporting Information Fig. 1), the biological relevance is arguable due to the small effect sizes (all differences were smaller than 5% of the overall mean). All later key analyses are based on qualitative measures independent of swim speed.
Behavioral Activity and Adult Hippocampal Neurogenesis Modulate the Use of Hippocampus‐Dependent Spatial Search Strategies
At each time point in the course of learning the water maze mice have a specific probability to choose an effective, more hippocampus‐dependent spatial search strategy that depends on the already learned spatial knowledge over a less hippocampus‐dependent nonspatial strategy (Redish, 1999). As in our previous study we assessed the qualitative aspects of spatial learning by classifying the search patterns using a parameter‐based algorithm (Balschun et al., 2003; Garthe et al., 2009; Ruediger et al., 2011) (for exact parameters of trial classification see Supporting Information). We asked whether RUN and ENR would affect the use of spatial versus nonspatial search strategies. Both ENR and RUN were contrasted with the additional condition of suppressed adult neurogenesis, denoted with the group suffix “TMZ.”
To estimate the effects of RUN, ENR, and TMZ on strategy use, all swim paths were classified into an either less hippocampus‐dependent or a more hippocampus‐dependent class of search strategies (see schematic in Figs. 3a,b for classification results). Using a logistic regression model, we statistically assessed changes in the chance (odds) for using either a more hippocampus‐dependent or less hippocampus‐dependent strategy under different experimental conditions. Specifically, we analyzed the effects of absent neurogenesis (TMZ, ENR + TMZ, and RUN + TMZ groups) contrasted with either ENR or RUN conditions. For both ENR and RUN the regression model included the respective nonstandard condition and suppression of adult neurogenesis, either separately or combined, as well as housing under control conditions.
Figure 3.
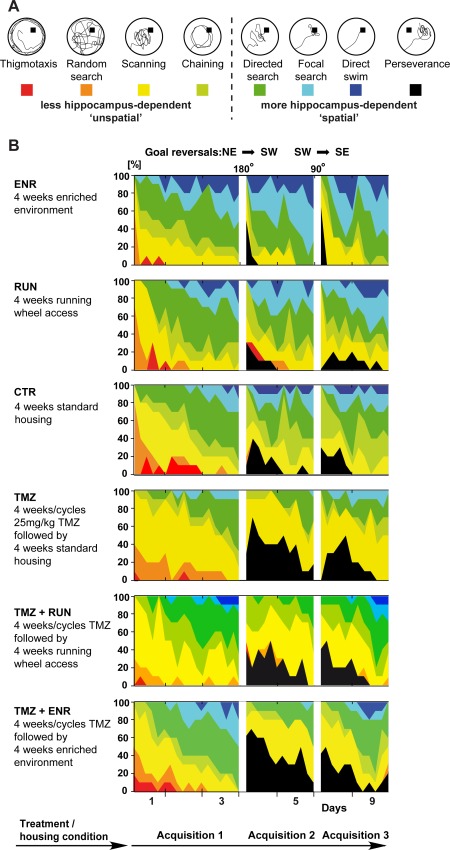
Algorithm‐based strategy classification. (a) Examples of search strategies used for classification. (b) Contribution of respective search strategies to group performance. Color‐code as indicated in (a). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For the ENR condition we found a statistically significant effect on strategy choice of both ENR and the treatment with TMZ, indicating the absence of adult neurogenesis (TMZ, Fig. 4a). Specifically, the estimated odds‐ratio (OR) for mice exposed to a stimulus‐rich environment compared with standard housing conditions was OR = 1.45 (P < 0.001), while suppression of adult neurogenesis resulted in an estimated OR = 0.73 (P < 0.001). Thus, living under ENR conditions significantly increased the chance of an animal to use a spatial, more hippocampus‐dependent strategy, while TMZ significantly decreased the chance to do so (OR = 1 would indicate no effect). Additionally, we found that with respect to strategy selection ENR and TMZ acted as statistically independent variables, i.e. there was no significant interaction effect between ENR and TMZ (P = 0.1528). This means that the OR of the combination treatment can be obtained by a simple multiplication of the individual ORs.
Figure 4.
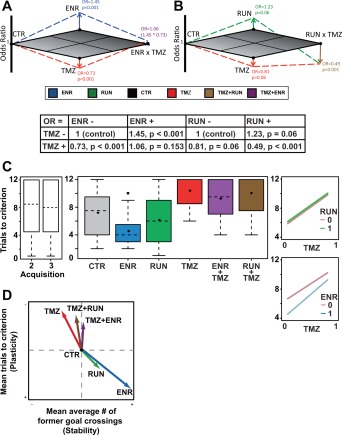
Differential effects of RUN, ENR, and TMZ on water maze performance. (a) and (b) visualize the effects of ENR, RUN, and TMZ (as determined by logistic regression analyses) in terms of the induced changes in the odds to choose a more hippocampus‐dependent strategy compared with controls. The interaction of ENR and TMZ was shown to be not statistically significant (P = 0.153). Therefore, the OR for applying ENR + TMZ can be calculated directly by multiplication of the ENR and the TMZ odds ratio. (c) Goal‐related plasticity indicated by the number of trials needed to regain the average path length shown on day 3 following subsequent goal reversals. From left to right: comparison of the distribution of the number of trials to criterion for probe trials 1 and 2; distribution of the number of trials to criterion for ENR, RUN, TMZ, and CTR (pooled over both probe trials), and the corresponding interaction plots (for explanation of box‐ and interaction plots see legend of Fig. 2). (d) Combines the results shown in Figures 2b and 4c to illustrate the respective shifts in the stability versus plasticity trade‐off. The number of goal crossings is interpreted as representing a successful encoding of the platform's position and thus “stability,” while the ability to re‐learn following goal reversal is taken as “plasticity.” [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For the RUN condition (Fig. 4b) the logistic regression identified a different effect constellation. There were only robust trends for the effects of RUN (OR = 1.23, P = 0.06) or TMZ (OR = 0.81, P = 0.06) variation. Interestingly, strategy choice was affected by RUN in a similar direction as found for ENR conditions. However, we also found a significant interaction effect of RUN and TMZ with an OR = 0.49 (P < 0.001). In contrast to the ENR situation, here the combination of RUN + TMZ resulted in a clear decrease in the chance to choose a spatial strategy (OR = 0.49, P < 0.001). These results suggest that the TMZ effect is more pronounced if acting on top of the RUN condition.
For standard housing, the odds ratio for using more versus less hippocampus‐dependent search strategies was not significantly different from a 1:1 chance, i.e. an odds of 1 (odds = 1.12, P = 0.08/odds = 1.06, P = 0.4363 for ENR and RUN conditions, respectively), suggesting that this condition had no effect on the chance for choosing more hippocampus‐dependent and less dependent strategies.
In summary, ENR as a pro‐neurogenic condition improved task acquisition by facilitating the use of spatial search strategies, whereas suppression of adult neurogenesis compromised learning the water maze. Supporting our hypothesis, the observed gain‐ and loss‐of‐function effects appeared to be specific for domains of water maze learning that we had previously found to be impaired after suppression of adult neurogenesis (Garthe et al., 2009, 2014). Showing a robust trend only, the effect of RUN did not reach significance in our study. However, the strong interaction effect exclusively found for RUN + TMZ but not for ENR + TMZ implied a role of physical exercise and presumably its effects on adult neurogenesis in applying effective, more hippocampus‐dependent search strategies. At the same time the interaction underlines the relevance of nonhippocampal brain functions on water maze performance. This is also well illustrated by the fact that, regarding strategy selection, ENR‐TMZ mice performed as well as controls despite their lack of new neurons. In those mice the neurogenesis‐independent effects of ENR rescued the phenotype.
Exposure to a Stimulus‐Rich Environment Provides a Superior Goal‐Related Plasticity That Depends on Adult Neurogenesis
Suppressing adult neurogenesis results in impaired reversal learning (Garthe et al., 2009; Swan et al., 2014). After moving the goal position to another quadrant of the pool, TMZ‐treated animals continued searching at the previous goal position for many trials. This phase of perseverance characteristically includes a resampling phase, in which the animals revert to less directed strategies. Therefore, we here asked how many trials it took the mice after goal reversal to regain a mean path length that was equal or shorter than on the last day of the preceding acquisition. This value is a measure of “full recovery from perseverance” and tries to capture one central idea of functional plasticity.
Using this measure, after goal reversal only ENR but not RUN showed a significantly faster regain of initial performance levels compared with controls (ENR: P = 0.004, RUN: P = 0.66, Fig. 4c). In contrast, TMZ treated mice needed significantly more trials to regain the path lengths reached in previous acquisitions (P < 0.001).
Thus, also when multiple goals have to be learned in the same general context, ENR and TMZ affect the animals’ goal‐related flexibility in a complementary manner. The absence of an interaction between ENR and TMZ, however, suggests, that while new hippocampal neurons are necessary for the full ENR effect, ENR also influences goal‐related plasticity by other factors that are likely responsible for the neurogenesis‐independent effects of ENR in other settings (Irvine et al., 2006; Segovia et al., 2008, 2010).
DISCUSSION
In this study we show that living in a stimulus‐rich, cognitive challenging environment improves water maze learning and that the benefits to a relevant extent depend on adult hippocampal neurogenesis. Since an additional histological assessment of changes in the stem cell niche at all time points related to certain phases of spatial learning is not possible and a parallel histological study would still not solve the fundamental problem, while leading to exploding numbers of animals to be analyzed, the present study focused on behavioral parameters as the analytical endpoints. The conclusions related to adult neurogenesis are inferred. The effects found, however, either reproduced findings from previous studies or appeared in a complementary manner by affecting specific functional aspects of water maze performance.
While voluntary wheel running increases the proliferation of precursor cells and thus the potential of new neurons that can be recruited into the network of the dentate gyrus, we demonstrate that only ENR facilitated learning performance in certain aspects of the water maze task in a presumably neurogenesis‐dependent manner. Specifically, ENR increased the chance of using more hippocampus‐dependent and effective search strategies for locating the hidden goal, allowed a superior probe trial performance, and increased the animals’ ability to cope with reversal situations. These effects were absent, when the animals were treated with TMZ in order to suppress adult neurogenesis. Although there was a trend towards an improved overall performance for the RUN condition, we did not find significant effects of RUN on strategy‐use, performance in probe trials, and reacquisition following goal reversals. This result should not be interpreted in the sense that exercise does not affect behavioral performance through changes in adult neurogenesis: for such strong conclusion the basis of information is too slim. However, the direct comparison between RUN and ENR reveals, not surprisingly, that the more cognitive stimulus has a stronger and more effect on performance. Within the concept of “leading an active life” physical activity plays a central role that ethologically is tightly linked to cognitive performance (see discussion in Kempermann, 2012; Freund et al., 2013).
The previous demonstration that animals showed improved ability to separate different visual contexts, when more new neurons were present in the dentate gyrus, had confirmed theoretical predictions (Clelland et al., 2009; Sahay et al., 2011), but the question was still open whether the successful differentiation of contexts is also relevant in the water maze as an example of a more naturalistic spatial learning task involving the hippocampus. Our loss‐ and gain‐of‐function experiments indicate that adult neurogenesis is relevant for specific aspects of spatial learning in the water maze, but also that the presence of the new neurons alone does not explain all aspects of improved learning performance after the intervention. This is in line with our hypothesis that adult neurogenesis provides an added functionality to the hippocampus but is not required for baseline performance. It might therefore be misleading, if in the context of adult neurogenesis one focuses on key functions such as “pattern separation.” Adult neurogenesis improves the quality and flexibility of the response but is not the only relevant mechanism for “pattern separation.” The water maze is an implementation of a situation requiring constant separations of context. Note that the use of the term “pattern separation” is not uniform in the literature: we use it here in the broader sense (also called “behavioral pattern separation”), not the stricter computational one referring to the orthogonalization of neuronal inputs.
Learning means to adapt behavior to specific contexts to master the challenges imposed by that situation and to make predictions for the future. In the water maze, the challenge is to navigate effectively toward a hidden platform. Contexts are provided by unique configurations of visual landmarks outside the pool. These contextual configurations allow associating specific areas of the water surface within the perimeter that is defined by the pool with a distinct directionality of the movement that brings the animal closer to the target zone. In essence, thus, efficient navigation depends on the flexible use of previously learned contexts. Consequently, the successful differentiation of contexts facilitates the use of directed and efficient search strategies.
Kee et al. demonstrated, that the population of those 5‐ to 8‐week‐old new neurons becomes preferentially recruited in water maze learning (Kee et al., 2007). We propose that ENR mice showed both a significantly higher probability to use efficient search strategies and a superior memory for the previously learned goal location because the greater number of new, plastic neurons after ENR improved their ability to differentiate contexts. This would allow using more efficient strategies to find the hidden goal and applying those strategies more flexibly, which is an ability already shown to be dependent on adult neurogenesis (Dupret et al., 2008).
Contexts are not solely defined by specific cue configurations but also by more task‐related parameters such as the actual goal position (Garthe and Kempermann, 2013). Following goal reversal, already known places (defined by external cue configurations) that have been associated with the first goal position now have to be interpreted in the context set by the new goal position. Successful differentiation of contexts remains important also on higher contextual levels defined by changing goal positions. Therefore, we further propose that because more plastic new neurons were available to mice reared under ENR conditions, their ability to differentiate contexts related to specific goals was increased, resulting in a superior ability to cope with changed platform positions. Importantly, this does not imply that all positive effects of ENR are mediated by adult neurogenesis alone.
Our hypothesis is supported by the strong interaction effect between ENR and TMZ found for the number of former goal crossings, suggesting a major functional contribution of adult neurogenesis to the ENR effect on this parameter. However, the absence of such an interaction for other parameters does not rule out additional involvements of new hippocampal neurons, as TMZ specifically prevented most of the beneficial effects of ENR. While adult neurogenesis appears to be highly relevant for certain aspects of spatial learning, the actual contribution of the new neurons considerably changes according to the specific task‐related demands present at a respective stage within the learning process, that is, precise knowledge of a specific location (goal crossings) versus route planning abilities and execution (directional aberration, strategy‐selection). In the same way, living in a stimulus‐rich environment affects brain function at all levels, adult neurogenesis in the hippocampus being only one among others.
In contrast to ENR, voluntary wheel running primarily affects Type‐2 precursor cell proliferation and thus increases the number of new cells that potentially can become new plastic neurons (van Beusekom et al., 1981; Van Praag et al., 1999; Steiner et al., 2008). On the other hand, effects on later stages of development do not appear to be independent of the potential raised by RUN (Fabel et al., 2009). It has even been claimed that physical activity would represent the determining component of the ENR effects on neurogenesis (Kobilo et al., 2011), including on water maze performance (Mustroph et al., 2012). This is not what we see.
In a recent study we found that in an ENR setting plain locomotion explains less variance in adult neurogenesis than exploration‐ and experience‐driven territorial coverage (“roaming entropy”). In our eyes, this qualitative component underscores the importance of a cognitive stimulating and challenging environment for recruiting the new neurons (Freund et al., 2013). In line with this, in the present study the chance of RUN mice to choose efficient spatial search strategies was not significantly different from controls. Furthermore, a better memory for the previously learned platform position as well as an increased goal‐related functional plasticity as found for ENR mice, was absent in the RUN group.
Still, there is a neurogenesis‐related effect of RUN on water maze performance as shown by the strong, statistically significant interaction with TMZ in strategy selection. The effect of TMZ was even stronger, when mice were exposed to TMZ and RUN at the same time, compared with TMZ alone. Since it is the newborn immature neurons that provide plasticity to the network of the dentate gyrus and thus contribute a specific functional role (Aimone et al., 2006; Deng et al., 2010), RUN might actually lead the hippocampus to “expect” new immature neurons to become available.
Although adult neurogenesis seems to be involved in several stages of learning the water maze task, the degree to which different parameters actually appear to depend on the new neurons appears to vary considerably. While the process of initial acquisition also includes the formation of a cognitive map as a supposedly primary hippocampal contribution, an animal also has to learn the tasks general constraints, the appearance, and reliability of distant visual cues, as well as to develop appropriate behavioral strategies to use the cognitive map effectively.
Thus, while the presence of new hippocampal neurons appeared to implicitly influence nearly all parameters associated with successful navigation, other factors such as general arousal levels, dendritic branching and sprouting, or efficiency of synaptic transmission turned out to be at least equally important. ENR is known to affect many of these factors (Irvine et al., 2006; Segovia et al., 2008, 2010), adult neurogenesis being one of them.
In line with this, we found the effects of ENR and TMZ on strategy selection to be independent. ENR attenuated the negative effects of suppressed adult neurogenesis, although the positive effect seen with ENR alone was missing. Thus, while ENR still improved some aspects of water maze performance without new hippocampal neurons, the full gain‐of‐function effect appeared to be dependent on adult neurogenesis.
Theoretical models have linked adult hippocampal neurogenesis to the stability‐plasticity dilemma (Wiskott et al., 2006). The stability‐plasticity dilemma refers to situations where new information has to be stored in a network of limited size along with highly similar, already stored contents. Preserving old memories should not prevent new items from being encoded and stored in the same network.
Our results show that only exposure to the ENR condition together with adult neurogenesis allows solving the stability‐plasticity dilemma, as mice from the ENR group showed a higher number of precise goal crossings during probe trials as well as a superior ability to cope with goal reversals. This can be illustrated by combining the graphs shown in Figures 2b and 4c into a new display, in which the arrows indicate the respective shifts in the stability and plasticity trade‐off for the different living conditions tested in our study with and without new hippocampal neurons (Fig. 4d). While the number of goal crossings is interpreted as representing a successful encoding of the platform's position and thus “stability,” the ability to relearn following goal reversal is supposed to reflect “plasticity.”
In conclusion, our data indicate that in mice adult neurogenesis can significantly improve specific aspects of hippocampus‐dependent spatial learning that reflect the task‐related demands of the respective stage within the overall learning process.
We here focused on behavioral parameters as the analytical endpoints to reveal how RUN and ENR affect spatial learning over multiple acquisition periods and including two goal reversals. Although the specific and dynamic interactions of defined aspects of spatial learning and precursor cell proliferation as well as the differentiation and survival of new neurons in the dentate gyrus will have to be addressed by future studies, our data provide suggestive evidence for a specific contribution of adult neurogenesis to water maze learning. While we could demonstrate in a previous study specific functional deficits following suppression of adult neurogenesis, in the present study these findings could not only be confirmed but extended by describing complementary gain‐of‐function effects in the same functional aspects of spatial learning. The fact that these specific functional improvements could only be observed following ENR experience correspond with results of numerous studies describing an increased survival of new hippocampal neurons and thus increased new neuron numbers. The extent, to which the new neurons actually affected spatial learning, appeared to depend on a preceding exposure to a stimulus‐rich environment.
It is interesting to speculate how living under ENR conditions actually exerts its effects on the new hippocampal neurons. Recent studies suggest the MAPK pathway as a common mediator underlying both learning and memory formation and ENR‐induced improvements of cognition: MSK1‐KO mice did not show functional improvement following exposure to a stimulus‐rich environment (Karelina et al., 2012). Pan et al. have shown that deletion of ERK5 as another member of the MAP kinase family inhibited adult neurogenesis accompanied by learning and memory deficits that appeared highly similar to those observed in mice lacking new neurons after TMZ treatment (Pan et al., 2012).
In turn, activation of endogenous ERK5 increased adult neurogenesis and hippocampus‐dependent learning and memory in the water maze and other spatial tasks (Wang et al., 2014). Living in a stimulus‐rich environment leads to activation of the MAPK pathway by increased growth factor levels, cytokine stimulation, or oxidative stress. In this view a direct activation of ERK5 could mimic some aspects of ENR, although this likely misses the full range of effects caused by the interaction of learning brains with a complex environment reflecting dynamically changing task demands.
Living in a complex environment represents a significant cognitive challenge. Adult neurogenesis helps to optimize the hippocampal network and thus improves an animals’ ability to acquire adequate and efficient behaviors (Freund et al., 2013). Partly depending on adult neurogenesis ENR improved aspects of spatial learning that likely relate to cognitive challenges that are relevant for survival under more naturalistic conditions. Accordingly, at an evolutionary level we have hypothesized that new neurons would facilitate adaptability to changing habitats. (Kempermann, 2012).
This argument gains specific importance when adult neurogenesis is considered in its potential role in modulating hippocampal function over the full lifespan resulting in what we have referred to as a “neurogenic reserve” (Kempermann, 2008).
Our present findings support our idea that adult neurogenesis is a key component of activity‐dependent strategies to preserve cognitive flexibility in aging.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
REFERENCES
- Aimone JB, Wiles J, Gage FH. 2006. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9:723–727. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. 2009. Computational influence of adult neurogenesis on memory encoding. Neuron 61:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez‐Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. 2014. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344:598–602. [DOI] [PubMed] [Google Scholar]
- Appleby PA, Kempermann G, Wiskott L. 2011. The role of additive neurogenesis and synaptic plasticity in a hippocampal memory model with grid‐cell like input. PLoS Comput Biol 7:e1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU, Lipp HP. 2003. Does cAMP response element‐binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus‐dependent memory? J Neurosci 23:6304–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD,J, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. 2010. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. 2008. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3:e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Galicia PL, Kempermann G. 2009. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci 3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Kruger A, Sachser N, Lindenberger U, Kempermann G. 2013. Emergence of individuality in genetically identical mice. Science 340:756–759. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. 2009. Adult‐generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One 4:e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Huang Z, Kaczmarek L, Filipkowski RK, Kempermann G. 2014. Not all water mazes are created equal: Cyclin D2 knockout mice with constitutively suppressed adult hippocampal neurogenesis do show specific spatial learning deficits. Genes Brain Behav 13:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Kempermann G. 2013. An old test for new neurons: Refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci 7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine GI, Logan B, Eckert M, Abraham WC. 2006. Enriched environment exposure regulates excitability, synaptic transmission, and LTP in the dentate gyrus of freely moving rats. Hippocampus 16:149–160. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD,J, Consiglio A, Lie DC, Squire LR, Gage FH. 2009. Dentate gyrus‐specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem 16:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Hansen KF, Choi YS, DeVries AC, Arthur JS, Obrietan K. 2012. MSK1 regulates environmental enrichment‐induced hippocampal plasticity and cognitive enhancement. Learn Mem 19:550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. 2007. Preferential incorporation of adult‐generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10:355–362. [DOI] [PubMed] [Google Scholar]
- Kempermann G. 2008. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci 31:163–169. [DOI] [PubMed] [Google Scholar]
- Kempermann G. 2012. New neurons for ‘survival of the fittest’. Nat Rev Neurosci 13:727–736. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. 2011. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem 18:605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. 2003. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467:455–463. [DOI] [PubMed] [Google Scholar]
- Martinez‐Canabal A, Akers KG, Josselyn SA, Frankland PW. 2013. Age‐dependent effects of hippocampal neurogenesis suppression on spatial learning. Hippocampus 23:66–74. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. 2012. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience 219:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niibori Y, Yu TS, Epp JR, Akers KG, Josselyn SA, Frankland PW. 2012. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun 3:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Anderson ML, Shors TJ. 2012. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci 36:3521–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YW, Chan GC, Kuo CT, Storm DR, Xia Z. 2012. Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen‐activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J Neurosci 32:6444–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. 1999. Beyond the Cognitive Map: From Place Cells to Episodic Memory. London: MIT Press. [Google Scholar]
- Ruediger S, Vittori C, Bednarek E, Genoud C, Strata P, Sacchetti B, Caroni P. 2011. Learning‐related feedforward inhibitory connectivity growth required for memory precision. Nature 473:514–518. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, de Blas M, Garrido P, Mora F. 2008. Effects of an enriched environment on the release of dopamine in the prefrontal cortex produced by stress and on working memory during aging in the awake rat. Behav Brain Res 187:304–311. [DOI] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, De Blas M, Garrido P, Mora F. 2010. Environmental enrichment increases the in vivo extracellular concentration of dopamine in the nucleus accumbens: A microdialysis study. J Neural Transm 117:1123–1130. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. 2005. A role for adult neurogenesis in spatial long‐term memory. Neuroscience 130:843–852. [DOI] [PubMed] [Google Scholar]
- Steiner B, Zurborg S, Horster H, Fabel K, Kempermann G. 2008. Differential 24 h responsiveness of Prox1‐expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid‐induced seizures. Neuroscience 154:521–529. [DOI] [PubMed] [Google Scholar]
- Swan AA, Clutton JE, Chary PK, Cook SG, Liu GG, Drew MR. 2014. Characterization of the role of adult neurogenesis in touch‐screen discrimination learning. Hippocampus 24:1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. 2007. Experience‐specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci 27:3252–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusekom HJ, van de Putte LB, van den Berg WB, van den Broek WJ. 1981. Antigen‐induced arthritis: Inflammation and antigen handling. Scand J Rheumatol Suppl 40:37–41. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270. [DOI] [PubMed] [Google Scholar]
- Wang W, Pan YW, Zou J, Li T, Abel GM, Palmiter RD, Storm DR, Xia Z. 2014. Genetic activation of ERK5 MAP kinase enhances adult neurogenesis and extends hippocampus‐dependent long‐term memory. J Neurosci 34:2130–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. 2006. Inhibition of neurogenesis interferes with hippocampus‐dependent memory function. Hippocampus 16:296–304. [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ, Kempermann G. 2006. A functional hypothesis for adult hippocampal neurogenesis: Avoidance of catastrophic interference in the dentate gyrus. Hippocampus 16:329–343. [DOI] [PubMed] [Google Scholar]
- Woolley DG, Vermaercke B, Op de Beeck H, Wagemans J, Gantois I, D'Hooge R, Swinnen SP, Wenderoth N. 2010. Sex differences in human virtual water maze performance: Novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res 208:408–414. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. 2008. A role for adult TLX‐positive neural stem cells in learning and behaviour. Nature 451:1004–1007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information


