Scheme 2. Synthesis of (A) the Thiazolin–Thiazole 17, (B) the Thiazole–Thiazoline 23, and (C) the Bithiazole 27a.
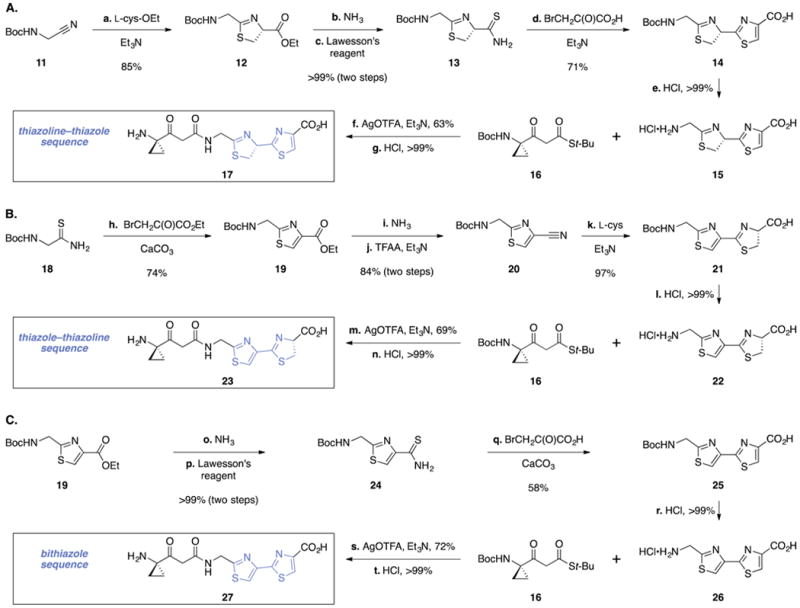
aReagents and conditions: (a) L-(+)-cysteine ethyl ester hydrochloride, Et3N, CH3OH, 23 °C, 85%; (b) NH3, CH3OH–H2O (2:1), 23 °C, >99%; (c) Lawesson’s reagent, CH2Cl2, 23 °C, >99%; (d) bromopyruvic acid, Et3N, CH3OH, reflux, 71%; (e) HCl, CH2Cl2–1,4-dioxane (4:1), 23 °C, >99%; (f) silver trifluoroacetate (AgO(TFA)), Et3N, DMF, 0 °C, 63%; (g) HCl, CH2Cl2–1,4-dioxane (4:1), 23 °C, >99%; (h) ethyl bromopyruvate, CaCO3, EtOH, 23 °C, 74%; (i) NH3, CH3OH–H2O (2:1), 23 °C, >99%; (j) trifluoroacetic anhydride (TFAA), Et3N, CH2Cl2, 0 → 23 °C, 84%; (k) L-(+)-cysteine, Et3N, CH3OH, reflux, 97%; (l) HCl, CH2Cl2–1,4-dioxane (8:1), 23 °C, >99%; (m) AgO(TFA), Et3N, DMF, 0 °C, 69%; (n) HCl, CH2Cl2–1,4-dioxane (4:1), 23 °C, >99%; (o) NH3, CH3OH–H2O (2:1), 23 °C, >99%; (p) Lawesson’s reagent, CH2Cl2, 23 °C, >99%; (q) bromopyruvic acid, CaCO3, EtOH, 23 °C, 58%; (r) HCl, CH2Cl2–1,4-dioxane (4:1), 23 °C, >99%; (s) AgO(TFA), Et3N, DMF, 0 °C, 72%; (t) HCl, CH2Cl2–1,4-dioxane (4:1), 23 °C, >99%.
