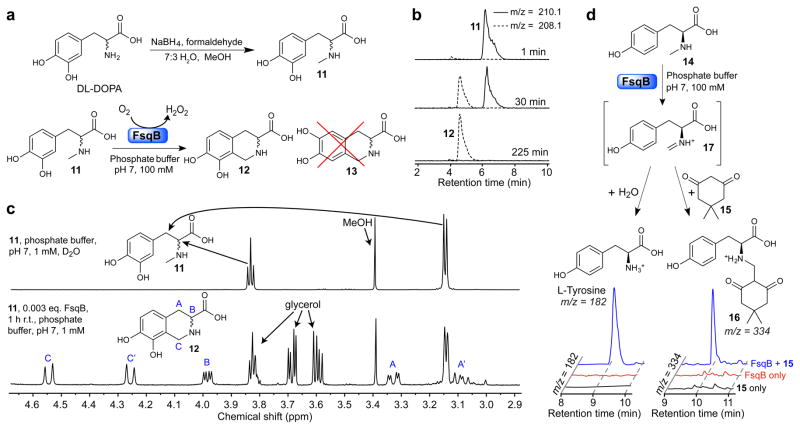
Figure 5. Enzymatic activity of recombinant FsqB.
(a) Synthesis of N-methyl-3′,4′-dihydroxy-DL-phenylalanine 11 (top) and regioselective conversion of 11 into 12 by purified recombinant FsqB (bottom). The isomer 13 does not form. (b) Ion chromatograms at indicated timepoints for the FsqB-catalyzed in vitro conversion of 11 to 12 (100 mM phoshate buffer, pH 7, 100:1 substrate to enzyme). (c) 1H NMR spectra of a sample of 11 (1 mM phosphate buffer in D2O) prior to FsqB addition (top) and 1 h post FsqB addition (333:1 substrate to enzyme), showing selective conversion of 11 into 12 (bottom) (d) Ion chromatograms showing formation of tyrosine and 16 as a result of capture of intermediate 17 by H2O or dimedone, 15 (1.5 μM FsqB and 400 μM N-methyl-L-tyrosine in 100 mM phoshate buffer, pH 7).