Abstract
S. aureus and S. pneumoniae are both common pathogens that are also carried by a large proportion of healthy individuals in the nasal and nasopharyngeal spaces. A negative association between carriage of S. aureus and S. pneumoniae has been reported in children in various epidemiologic studies from different geographical regions. Most studies found that the negative association between S. pneumoniae and S. aureus was significant only for carriage of vaccine-type S. pneumoniae strains. In this review, we summarize the various suggested mechanisms of this suggested bacterial interference, and the clinical implications reported following PCV introduction to date in various geographical regions.
Introduction
S. aureus and S. pneumoniae are both common pathogens that are also carried by a large proportion of healthy individuals in the nasal and nasopharyngeal spaces. S. aureus is a major source of morbidity and mortality worldwide, with infections ranging from minor skin infections to invasive infections such as endocarditis and toxic shock syndrome. It is persistently carried by approximately 25% of the healthy population.1 As with S. pneumoniae, carriage of S. aureus serves as the first step to infection as well as the frequent source of transmission between one individual to another.
Over a decade ago, two studies independently reported a negative association between carriage of S. aureus and S. pneumoniae.2,3 During the following years multiple epidemiological studies in different geographical regions observed similar findings of a negative association between carriage of S. pneumoniae and S. aureus in young children.4-8 The carriage of both species was associated with age, with the peak S. pneumoniae carriage and lowest S. aureus carriage at 6 months to 3 years4-10 and peak S. aureus colonization at age <6 months and 5–7 y11 The negative association was significant even after adjusting for age, but this interference was not observed in older children and adults.8,12 Interestingly, most studies2,3,5,9 found that the inverse correlation between S. pneumoniae and S. aureus was significant only for carriage of vaccine-type S. pneumoniae strains, which were carried more commonly before the introduction of the pneumococcal vaccine.
This finding, together with an earlier clinical trial that reported increased S. aureus otitis media following PCV vaccination,13 raised much concern3,7,14,15; if S. pneumoniae carriage protects from S. aureus carriage and the introduction of the pneumococcal conjugate vaccines (PCV) results in decreased S. pneumoniae carriage, this could potentially lead to an increase in S. aureus carriage, and infection. In this review, we summarize the various suggested mechanisms of this inverse correlation, and the clinical implications reported following PCV introduction to date in various geographical regions.
Suggested Mechanisms of Interaction between S. pneumoniae and S. aureus
As with any bacterial interaction, the association between S. pneumoniae and S. aureus can theoretically be caused by either direct or indirect interactions. Direct interactions, such as direct competition for adhesion sites, resources and receptor-mediated interactions are unlikely in this case due to the fact that the 2 bacteria reside in closely located, yet different niches. Interactions through secreted factors are more likely, as well as indirect interactions mediated through other bacteria, or through the immune system.
Suppression of S. aureus by H202 production by S. pneumoniae
Hydrogen peroxide produced by S. pneumoniae was first postulated to have a role in the inhibition of S. aureus and other respiratory pathogens by Mcleod and Gordon in 1922.16 Nearly a century later Pericone et al. observed that H202 in S. pneumoniae culture supernatant was bactericidal against H. influenza and N. meningitidis, and to a lesser extent against M. catarrhalis.17 Regev-Yochay et al found that the in vitro bactericidal activity of S. pneumoniae toward S. aureus is indeed mediated through hydrogen peroxide; The bactericidal effect was reversible with catalase and S. pneumoniae spxB mutants that do not produce H202 were not bactericidal.18 Following this observation, Selva et al. suggested that the mechanism of interference is activation of S. aureus resident prophages by low levels of hydrogen peroxide produced by S. pneumoniae,19 which then lyse S. aureus cells.
In vivo murine studies that assessed this issue are conflicting. In line with the theory of H202 interference, Park et al demonstrated that S. aureus catalase expression contributes to its ability to colonize and survive in the presence of S. pneumoniae in an in vivo mouse model of nasal co-colonization.20 However, in a neonatal rat model, Margolis observed that S. aureus density did not differ whether co-colonized with hydrogen peroxide producing or non-producing S. pneumoniae, or whether catalase or non-catalase producing S. aureus strains were tested.21
To assess the role of hydrogen peroxide in the patterns of human co-colonization, Regev-Yochay et al. assessed the variation of bactericidal activity in S. pneumoniae strains isolated from children co-colonized with S. pneumoniae and S. aureus compared to those colonized only with S. pneumoniae. They showed only a trend toward a negative correlation between co-colonizing S. pneumoniae strains and bactericidal activity and concluded that the variation in hydrogen-peroxide production alone does not fully explain the pattern of co-colonization.22
Genetic bacterial determinants of interference
Melles et al. assessed the possibility of a genotype-specific association between S. aureus and S. pneumoniae carriage and did not find such a correlation. They suggested that only more subtle genetic variations may possibly play a role in the interference between the two.23 In line with this, a study by Nouwen et al. showed that S. aureus carriage of a strain is not dependent on bacterial genotype, suggesting that it is instead related to host factors.24 Margolis et al. determined that the colonizing strain of S. aureus in a neonatal rat model is determined solely by which strain is first to colonize and not by the characteristics of that strain.25
Interactions with other residents of the upper respiratory tract microbiome
S. aureus and S. pneumoniae are not the only species present in the nasopharyngeal region. Over the years, many studies have observed interactions between various bacterial species and viruses carried in the upper respiratory tract.26-30 These bacteria and viruses compete for space and resources25,30,31 and in some cases, such as influenza virus and S. pneumoniae, the virus and bacteria act synergistically to cause increased S. pneumoniae adhesion to host cells.32
The most clinically relevant and commonly studied interactions and competition are those between the upper respiratory tract pathogens, namely S. pneumoniae, S. aureus, H influenzae, and M. catarrhalis. The prevalence of these pathogens varies between populations, but most children are colonized by at least one of these species in the first year of life.5,8,33-37 Positive correlations between S. pneumoniae, M. catarrhalis and H. influenza, and negative correlations between H. influenza and S. aureus have been reported in epidemiological studies5,7,8,38,39 as well as infection models.40,25,41 Pettigrew et al. showed that colonization with M. catarrhalis and H. influenza together doubled the likelihood of co-colonization with S. pneumoniae. 7
Recent advances in sequencing technology have allowed for the detection of the entire nasal microbiome, not only of culturable strains.42 Metagenomic analyses showed the presence of a highly diverse nasopharyngeal microbiome including up to 2042 observed operational taxonomic units and as many as 1,219,310 observed unique sequences.4,43,44 Considering the vast diversity in the nasal microbiome with which the two pathogens interact, the relationship between the two pathogens should take into consideration the possible roles of other species in the nasal microbiome, possibly by inhibiting or promoting the growth of S. aureus or S. pneumoniae.
In a study by Cremers et al., in which nasal microbiomes of S. pneumoniae carriers and non-carriers were assessed before and after artificial inoculation of non-carriers with S. pneumoniae, they observed that colonization was less likely to be successful if the individual's microbiome was rich in Staphylococcal species.45 In line with this, Laufer et al. observed that S. pneumoniae colonization was more frequently detected when the microbiome had lower population diversity.4
S. aureus has also been shown to affect and be affected by other species in the upper respiratory niche. Analysis of the nasal microbiome by Lina et al. found a negative association between Corynebacterium species and S. aureus carriage,28 and similarly, Yan et al. found that various species of Corynebacterium were either positively (C. accolens) or negatively (C. pseudodiphtheriticum) associated with the presence of S. aureus. Evidence for these interactions was seen in vitro44 and in vivo, as artificial inoculation of S. aureus carriers with a species of Corynebacterium was shown to eradicate a resident S. aureus species.46
Another bacteria that was also suggested to interact with S. aureus in the nose is Staphylococcus epidermidis. Iwase et al. showed that a serine protease produced by some S. epidermidis strains inhibited S. aureus biolfilm formation. These inhibitory strains were more likely to be isolated from individuals who did not carry S. aureus, while non- inhibitory strains were isolated from S. aureus carriers.47
The role of the immune system in the bacterial interference
While some studies focused on potential direct mechanisms of interference, i.e. competition for space and resources30,31,46 or direct inhibition through bactericidal factors,18,21,41,48 the fact that S. aureus and S. pneumoniae colonize closely related, yet not precisely the same region in the upper respiratory niche, suggests that indirect inhibition, such as immune mediated inhibition is more plausible. The concept of interspecies immune-mediated cross-reactivity is not new, with some examples being cowpox and small pox, as discovered by Edward Jenner,49 or certain enteric commensal Escherichia coli.50
Repeated S. pneumoniae carriage episodes in early childhood eventually induces an immune response that leads to shorter and less dense colonization of S. pneumoniae in older children and adults.51 In contrast, S. aureus appears to elicit a futile antibody response, allowing the bacteria to escape immune responses and recurrent colonization and infections with S. aureus are common.52-54 Moreover, ∼20% of healthy adult individuals are persistent carriers of a single strain for many years,55,56 while young healthy adults are typically carriers of S pneumoniae only for very short durations, even following experimental exposures.57 Shak et al. showed that when S. aureus carriers were artificially inoculated with S. pneumoniae, successful colonization resulted in a decrease in S. aureus carriage, but only 14 d later. The authors suggested that this delayed response may point to an immune mediated interaction.41
Initial suggestive data on the role of immune-mediated interference arose from studies that showed that S. pneumoniae – S. aureus interference was observed only in HIV uninfected children, but did not exist in HIV-infected individuals.58,59 Colonization rates with S pneumoniae alone have been shown to be the same60,61 or higher62 in HIV infected compared to uninfected children, and HIV-positive patients have been shown to carry a wider range of S. pneumoniae serotypes.63 In HIV treated children, the interference was once again observed as in uninfected children.64 Indeed, pneumococcal-specific CD4+ T cells have been found to return to normal levels following anti-retroviral therapy.65 This suggests that the interaction between the S. aureus and S. pneumoniae may be CD4+ T cell-mediated.
While the HIV studies may suggest that T-cell immune response plays a role in the interference, Lijek et al. demonstrated in an in vivo murine model that S. pneumoniae colonization inhibits subsequent S. aureus acquisition in an antibody-dependent manner, via cross-reactive antibodies targeting conserved dehydrogenases, 1-pyroline-5-carboxylate dehydrogenase (P5CDH) of S. aureus and a putative S. pneumoniae dehydrogenase: SP_1119.66 Yet, these antibodies have not been found in humans. In addition, other studies did not find correlations between levels of particular S. aureus or S. pneumoniae antibodies and rates of S. aureus colonization.67,68
S. pneumoniae pilus as a potential determinant in the interference mechanism
Another possible mechanism of immune-mediated interaction that has been suggested is through an immune response elicited toward the pneumococcal pilus. The pneumococcal pilus is a long filamentous structure that plays a role in host cell adhesion and pathogenesis and has also been shown to elicit an inflammatory immune response from the host.69,70 Vaccine-type strains were more likely to carry a pilus71 and indeed, following vaccine implementation, the rates of carriage of vaccine-type strains, including piliated ones, declined. However other piliated strains emerged, either by acquiring the pilus, or due to expansion and unmasking.72 Piliated pneumococcal strains have been found to be negatively associated with S. aureus carriage regardless of whether those strains were included in the PCV7 vaccination, but carriage of non-piliated strains had no significant correlation with carriage of S. aureus.72 It is therefore possible that the negative association between S. aureus and vaccine-type S. pneumoniae is due to the presence of the pilus, or a pilus-associated virulence factor. Since many of these new strains were then included in PCV13,73 we have yet to see whether new piliated strains will again emerge.
While different mechanisms for a potential interference have been suggested, indirect mechanisms seem more plausible. The current reported data suggests that immune mediated mechanisms elicited by S. pneumoniae either specifically or non-specifically, directly or through other members of the microbiome interfere with S. aureus carriage (Fig. 1). Further studies are required to determine whether cross-reactive antibodies, non-specific immune response elicited by a particular pneumococcal structure or other mechanisms can fully explain this bacterial interference.
Figure 1.
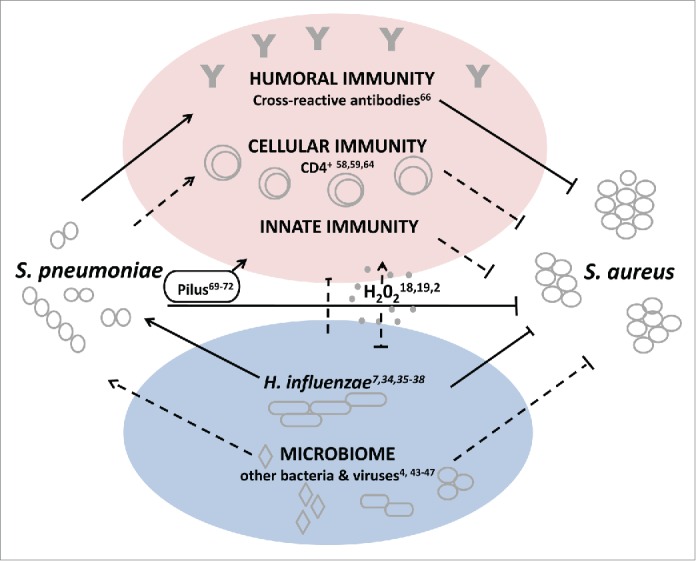
Model of possible mechanisms for S. aureus and S. pneumoniae interference. Straight lines indicate interactions that have been reported between S. aureus and S. pneumoniae. Dotted lines indicate interactions and/or directionality that have been suggested, but have not yet been observed experimentally.
Clinical Implications of the PCV effect on the Interference and on S. aureus Carriage
The discovery of the inverse correlation between S. aureus and vaccine-type strains of S. pneumoniae came at a time when PCV7 was just being introduced to the pediatric national immunization plan in many countries. Since vaccine-type strains were those found to be correlated with S. aureus carriage, this raised much concern that the introduction of the vaccine would indirectly cause a rise in S. aureus carriage and infection.7,11,14,15 The idea that external interventions in ecosystems could damage the natural equilibrium by eliminating a less virulent ‘predator’ and result in undesirable emergence of a possibly more virulent ‘prey’ is not new and was shown decades ago for pests and pesticides.74 The clinical trial that first suggested interference between S. pneumoniae and S. aureus found a rise in S. aureus otitis media cases following vaccination with PCV7 compared to a non-vaccinated group.13 Others alerted that the emergence in CA-MRSA in the USA may be related to the introduction of PCV7 which took place at the same time.75
Several studies have examined the dynamics of S. aureus carriage post-PCV introduction. Lee at al observed no changes in S. aureus carriage rates in children from Massachusetts 3 to 7 y following PCV7 implementation, but they did not compare their results to pre-vaccination S. aureus carriage rates. Interestingly, they also did not observe an inverse correlation between S. aureus and S. pneumoniae in their sample.11 The lack of observed interference is possibly due to the low rates of vaccine-type S. pneumoniae in their samples which were collected following PCV7 vaccination.11,76 The correlation could also be hidden in the sample due to the large age range of children in their study ranging from 3 months to 7 y Other studies that looked at older children, or looked at a wide range of children without differentiating between older and younger children did not see the individual or population level interference either,12,77 whereas those that only looked at children under the age of 2 y saw the interference more clearly.10 It is therefore difficult to interpret the results of this study regarding the effect of PCV7 on S. aureus carriage, particularly since they did not observe an inverse correlation throughout their study periods.
Two studies in the Netherlands observed temporary increases in S. aureus carriage in children aged 11–12 months following vaccination with PCV7.76,78 One of these was a randomized control vaccine trial done before the introduction of PCV7 in the Netherlands health program; the other was a cross-sectional surveillance study on nasopharyngeal bacterial carriage in the years following PCV7 introduction. The observed increase in S. aureus carriage coincides with a decrease in overall S. pneumoniae carriage, seen at age 11 months following vaccination with PCV7.76 In the same study population a higher bacterial diversity of the microbiome was observed among PCV7 vaccinated children aged 12 months compared to unvaccinated children.79 The relationship to age could be due to the fact that all 3 doses were needed in order to decrease carriage of S. pneumoniae strains, or it could be due to the immune maturation that occurs around age 12 months.80
Interestingly, the cross-sectional study reported increased parental S. aureus carriage concomitant to the rise of S. aureus in the child (at the age of 11 months).76 However, the randomized study did not observe an increase in parental carriage and surprisingly they observed decreased parental carriage when the child was 24 months old.78 A surveillance study from the pre-PCV era in South Africa reported constant maternal S. aureus colonization rates during times of dynamic S. aureus and S. pneumoniae carriage in the child.8 The seemingly conflicting results of these 3 studies may be explained by changes in carried serotypes in the population due to herd effects following widespread vaccination which are not observed in clinical trials.
PCV7 implementation did not result in long-term increases in S. aureus carriage and infection, including S. aureus otitis media and MRSA levels, as reported in studies done in countries around the world, including the USA, Netherlands, Israel, and China.7,11,76-78,81-84 Studies that observed increased S. aureus carriage in children age 11 months no longer saw this increase by age 24 months.76
Myth or Reality
The relationship between S. aureus and S. pneumoniae is very complex. The inverse correlation between carriage of S. aureus and carriage of vaccine-type S. pneumoniae in young children has been seen throughout many geographical areas, and through the years, including post-PCV7 implementation. However, the mechanism behind it and the clinical implications have yet to be fully determined.
Fears that vaccinating against S. pneumoniae would cause an increase in S. aureus, and more specifically MRSA carriage and infection seem, as of now, to be unsubstantiated. Changes following PCV7 introduction in overall S. aureus carriage rates were only short-term.
Carriage rates of both S. pneumoniae and S. aureus are dynamic. These two strains do not reside alone in the nose; their presence or absence can be affected by other species and other competitive factors, or external interventions such as vaccination or antibiotic use. It is still unclear whether the balance between the 2 species will continue to exist following widespread vaccination, and whether further vaccination with pneumococcal vaccines will cause a rise in S. aureus carriage and infection. It appears that the interaction between the two bacteria may be reduced due to vaccination, but the emergence of new strains and the evolution of existing strains makes it difficult to predict the implications. Many of the strains that replaced the vaccine-type strains following PCV7 implementation are now included in PCV13 and their prevalence rates are expected to decline as well. Complicating further, if piliated strains play a major role in the interference, determining the impact of PCVs on the prevalence of piliated strains will define the effect on S. aureus carriage.
Since the mechanism of interaction between the two species is not yet fully understood, it is impossible to predict whether the implementation of newer vaccines will result in further serotype replacement, a rise in S. aureus carriage, or a rise in a different species altogether. Clearly, further studies are required, both on the epidemiological effects of PCV vaccination, and on the mechanisms of this interaction.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Joseph Lewnard for critical reading and discussion in the preparation of this manuscript.
References
- 1.van Belkum A, Verkaik Nelianne J, de Vogel Corné P, Boelens Hélène A, Verveer J, Nouwen Jan L, Verbrugh Henri A, Wertheim Heiman FL. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 2009; 199:1820-6; PMID:19419332; http://dx.doi.org/ 10.1086/599119 [DOI] [PubMed] [Google Scholar]
- 2.Regev-Yochay G, Dagan R, Raz M, Carmeli Y, Shainberg B, Derazne E, Rahav G, Rubinstein E. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 2004; 292:716-20; PMID:15304469; http://dx.doi.org/ 10.1001/jama.292.6.716 [DOI] [PubMed] [Google Scholar]
- 3.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rümke HC, Verbrugh HA, Hermans PWM. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. The Lancet 2004; 363:1871-2; http://dx.doi.org/ 10.1016/S0140-6736(04)16357-5 [DOI] [PubMed] [Google Scholar]
- 4.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. Microbial communities of the upper respiratory tract and otitis media in children. mBio 2011; 2:e00245-10; PMID:21285435; http://dx.doi.org/ 10.1128/mBio.00245-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y-C, Chen C-J. Nasal carriage of methicillin-resistant Staphylococcus aureus during the first 2 years of life in children in Northern Taiwan. Pediatr Infect Dis J 2015; 34:131-5; PMID:25144800; http://dx.doi.org/ 10.1097/INF.0000000000000517 [DOI] [PubMed] [Google Scholar]
- 6.Lebon A, Labout JAM, Verbrugh HA, Jaddoe VWV, Hofman A, van Wamel W, Moll HA, van Belkum A. Dynamics and determinants of Staphylococcus aureus carriage in infancy: the generation R study. J Clin Microbiol 2008; 46:3517-21; PMID:18667593; http://dx.doi.org/ 10.1128/JCM.00641-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis 2008; 14:1584; PMID:18826823; http://dx.doi.org/ 10.3201/eid1410.080119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiri T, Nunes MC, Adrian PV, Van Niekerk N, Klugman KP, Madhi SA. Interrelationship of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus colonization within and between pneumococcal-vaccine naïve mother-child dyads. BMC Infect Dis 2013; 13:483; PMID:24134472; http://dx.doi.org/ 10.1186/1471-2334-13-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quintero B, Araque M, Van Der Gaast-de Jongh C, Escalona F, Correa M, Morillo-Puente S, Vielma S, Hermans P. Epidemiology of Streptococcus pneumoniae and Staphylococcus aureus colonization in healthy Venezuelan children. Eur J Clin Microbiol Infect Dis 2011; 30:7-19; PMID:20803226; http://dx.doi.org/ 10.1007/s10096-010-1044-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dukers-Muijrers N, Stobberingh E, Beisser P, Boesten R, Jacobs P, Hoebe C. Nasal carriage of Streptococcus pneumoniae serotypes and Staphylococcus aureus in Streptococcus pneumoniae-vaccinated and non-vaccinated young children. Epidemiol Infect 2013; 141:631-8; PMID:22687602; http://dx.doi.org/ 10.1017/S095026881200115X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee GM, Huang SS, Rifas-Shiman SL, Hinrichsen VL, Pelton SI, Kleinman K, Hanage WP, Lipsitch M, McAdam AJ, Finkelstein JA. Epidemiology and risk factors for Staphylococcus aureus colonization in children in the post-PCV7 era. BMC Infect Dis 2009; 9:110; PMID:19594890; http://dx.doi.org/ 10.1186/1471-2334-9-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito S, Terranova L, Ruggiero L, Ascolese B, Montinaro V, Rios WP, Galeone C, Principi N. Streptococcus pneumoniae and Staphylococcus aureus carriage in healthy school-age children and adolescents. J Med Microbiol 2015; 64:427-31; PMID:25614277 [DOI] [PubMed] [Google Scholar]
- 13.Veenhoven R, Bogaert D, Uiterwaal C, Brouwer C, Kiezebrink H, Bruin J, IJzerman E, Hermans P, de Groot R, Zegers B. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. The Lancet 2003; 361:2189-95; http://dx.doi.org/ 10.1016/S0140-6736(03)13772-5 [DOI] [PubMed] [Google Scholar]
- 14.Brogden KA, Guthmiller JM, Taylor CE. Human polymicrobial infections. The Lancet 2005; 365:253-5; http://dx.doi.org/ 10.1016/S0140-6736(05)70155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shak JR, Vidal JE, Klugman KP. Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol 2013; 21:129-35; PMID:23273566; http://dx.doi.org/ 10.1016/j.tim.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLeod JW, Gordon J. Production of hydrogen peroxide by bacteria. Biochem J 1922; 16:499-506; PMID:16743107; http://dx.doi.org/ 10.1042/bj0160499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pericone CD, Overweg K, Hermans PW, Weiser JN. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun 2000; 68:3990-7; PMID:10858213; http://dx.doi.org/ 10.1128/IAI.68.7.3990-3997.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regev-Yochay G, Trzciński K, Thompson CM, Malley R, Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol 2006; 188:4996-5001; PMID:16788209; http://dx.doi.org/ 10.1128/JB.00317-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selva L, Viana D, Regev-Yochay G, Trzcinski K, Corpa JM, Lasa í, Novick RP, Penadés JR. Killing niche competitors by remote-control bacteriophage induction. Proc Natl Acad Sci U S A 2009; 106:1234-8; PMID:19141630; http://dx.doi.org/ 10.1073/pnas.0809600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park B, Nizet V, Liu GY. Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J Bacteriol 2008; 190:2275-8; PMID:18223076; http://dx.doi.org/ 10.1128/JB.00006-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margolis E. Hydrogen peroxide-mediated interference competition by Streptococcus pneumoniae has no significant effect on Staphylococcus aureus nasal colonization of neonatal rats. J Bacteriol 2009; 191:571-5; PMID:19011027; http://dx.doi.org/ 10.1128/JB.00950-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regev-Yochay G, Malley R, Rubinstein E, Raz M, Dagan R, Lipsitch M. In vitro bactericidal activity of Streptococcus pneumoniae and bactericidal susceptibility of Staphylococcus aureus strains isolated from cocolonized versus noncocolonized children. J Clin Microbiol 2008; 46:747-9; PMID:18039795; http://dx.doi.org/ 10.1128/JCM.01781-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melles DC, Bogaert D, Gorkink RFJ, Peeters JK, Moorhouse MJ, Ott A, van Leeuwen WB, Simons G, Verbrugh HA, Hermans PWM, et al.. Nasopharyngeal co-colonization with Staphylococcus aureus and Streptococcus pneumoniae in children is bacterial genotype independent. Microbiology 2007; 153:686-92; PMID:17322188; http://dx.doi.org/ 10.1099/mic.0.2006/002279-0 [DOI] [PubMed] [Google Scholar]
- 24.Nouwen J, Boelens H, Van Belkum A, Verbrugh H. Human factor in Staphylococcus aureus nasal carriage. Infect Immun 2004; 72:6685-8; PMID:15501803; http://dx.doi.org/ 10.1128/IAI.72.11.6685-6688.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolis E, Yates A, Levin BR. The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response. BMC Microbiology 2010; 10:59; PMID:20178591; http://dx.doi.org/ 10.1186/1471-2180-10-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinefield HR, Wilsey JD, Ribble JC, Boris M, Eichenwald HF, Dittmar CI. Interactions of Staphylococcal colonization: influence of normal nasal flora and antimicrobials on inoculated Staphylococcus aureus strain 502A. Am J Dis Child 1966; 111:11-21; PMID:5900331; http://dx.doi.org/ 10.1001/archpedi.1966.02090040047002 [DOI] [PubMed] [Google Scholar]
- 27.Lipsitch M. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg Infect Dis 1999; 5:336-45; PMID:10341170; http://dx.doi.org/ 10.3201/eid0503.990304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lina G, Boutite F, Tristan A, Bes M, Etienne J, Vandenesch F. Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl Environ Microbiol 2003; 69:18-23; PMID:12513972; http://dx.doi.org/ 10.1128/AEM.69.1.18-23.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brook I, Gober AE. In vitro bacterial interference in the nasopharynx of otitis media–prone and non–otitis media–prone children. Arch Otolaryngol Head Neck Surg 2000; 126:1011-3; PMID:10922236; http://dx.doi.org/ 10.1001/archotol.126.8.1011 [DOI] [PubMed] [Google Scholar]
- 30.Murphy TF, Bakaletz LO, Smeesters PR. Microbial interactions in the respiratory tract. Pediatr Infect Dis J 2009; 28:S121-S6; PMID:19918134; http://dx.doi.org/ 10.1097/INF.0b013e3181b6d7ec [DOI] [PubMed] [Google Scholar]
- 31.Bibel DJ, Aly R, Bayles C, Strauss WG, Shinefield HR, Maibach HI. Competitive adherence as a mechanism of bacterial interference. Can J Microbiol 1983; 29:700-3; PMID:6411317; http://dx.doi.org/ 10.1139/m83-114 [DOI] [PubMed] [Google Scholar]
- 32.Avadhanula V, Rodriguez CA, DeVincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species-and cell type-dependent manner. J Virol 2006; 80:1629-36; PMID:16439519; http://dx.doi.org/ 10.1128/JVI.80.4.1629-1636.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis 1997; 175:1440-5; PMID:9180184; http://dx.doi.org/ 10.1086/516477 [DOI] [PubMed] [Google Scholar]
- 34.Vives M, Garcia ME, Saenz P, De Los Angeles Mora M, Mata L, Sabharwal H, Svanborg C. Nasopharyngeal colonization in Costa Rican children during the first year of life. Pediatr Infect Dis J 1997; 16:852-8; PMID:9306479; http://dx.doi.org/ 10.1097/00006454-199709000-00007 [DOI] [PubMed] [Google Scholar]
- 35.Faden H, Duffy L, Williams A, Krystofik DA, Wolf J. epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first 2 Years of life. J Infect Dis 1995; 172:132-5; PMID:7797903; http://dx.doi.org/ 10.1093/infdis/172.1.132 [DOI] [PubMed] [Google Scholar]
- 36.Faden H, Duffy L, Williams A, Krystofik D, Wolf J. Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first two years of life. Acta Otolaryngol Suppl 1996; 523:128-9; PMID:9082757 [PubMed] [Google Scholar]
- 37.De Lencastre H, Kristinsson KG, Brito-Avo A, Sanches IS, Sa-Leao R, Saldanha J, Sigvaldadottir E, Karlsson S, Oliveira D, Mato R. Carriage of respiratory tract pathogens and molecular epidemiology of Streptococcus pneumoniae colonization in healthy children attending day care centers in Lisbon, Portugal. Microb Drug Resist 1999; 5:19-29; PMID:10332718; http://dx.doi.org/ 10.1089/mdr.1999.5.19 [DOI] [PubMed] [Google Scholar]
- 38.Nzenze S, Shiri T, Nunes M, Klugman K, Kahn K, Twine R, de Gouveia L, von Gottberg A, Madhi S. Temporal association of infant immunisation with pneumococcal conjugate vaccine on the ecology of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus nasopharyngeal colonisation in a rural South African community. Vaccine 2014; 32:5520-30; PMID:25101982; http://dx.doi.org/ 10.1016/j.vaccine.2014.06.091 [DOI] [PubMed] [Google Scholar]
- 39.Jacoby P, Watson K, Bowman J, Taylor A, Riley TV, Smith DW, Lehmann D, Team KOMRP. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine 2007; 25:2458-64; PMID:17030494; http://dx.doi.org/ 10.1016/j.vaccine.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weimer KED, Armbruster CE, Juneau RA, Hong W, Pang B, Swords WE. Coinfection with Haemophilus influenzae promotes pneumococcal biofilm formation during experimental otitis media and impedes the progression of pneumococcal disease. J Infect Dis 2010; 202:1068-75; PMID:20715928; http://dx.doi.org/ 10.1086/656046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shak JR, Cremers AJH, Gritzfeld JF, de Jonge MI, Hermans PWM, Vidal JE, Klugman KP, Gordon SB. Impact of experimental human pneumococcal carriage on nasopharyngeal bacterial densities in healthy adults. PLoS One 2014; 9:e98829; PMID:24915552; http://dx.doi.org/ 10.1371/journal.pone.0098829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C. The NIH human microbiome project. Genome Res 2009; 19:2317-23; PMID:19819907; http://dx.doi.org/ 10.1101/gr.096651.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogaert D, Keijser B, Huse S, Rossen J, Veenhoven R, van Gils E, Bruin J, Montijn R, Bonten M, Sanders E. Variability and diversity of nasopharyngeal microbiota in children: A metagenomic analysis. PLoS One 2011; 6:e17035; PMID:21386965; http://dx.doi.org/ 10.1371/journal.pone.0017035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan M, Pamp Sünje J, Fukuyama J, Hwang Peter H, Cho D-Y, Holmes S, Relman David A. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe 2013; 14:631-40.; PMID:24331461; http://dx.doi.org/ 10.1016/j.chom.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cremers AJ, Zomer AL, Gritzfeld JF, Ferwerda G, van Hijum SA, Ferreira DM, Shak JR, Klugman KP, Boekhorst J, Timmerman HM. The adult nasopharyngeal microbiome as a determinant of pneumococcal acquisition. Microbiome 2014; 2:44; PMID:25671106; http://dx.doi.org/ 10.1186/2049-2618-2-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uehara Y, Nakama H, Agematsu K, Uchida M, Kawakami Y, Abdul Fattah ASM, Maruchi N. Bacterial interference among nasal inhabitants: eradication of Staphylococcus aureus from nasal cavities by artificial implantation of Corynebacterium sp. J Hosp Infect 2000; 44:127-33; PMID:10662563; http://dx.doi.org/ 10.1053/jhin.1999.0680 [DOI] [PubMed] [Google Scholar]
- 47.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010; 465:346-9; PMID:20485435; http://dx.doi.org/ 10.1038/nature09074 [DOI] [PubMed] [Google Scholar]
- 48.Wannamaker LW. Bacterial interference and competition. Scand J Infect Dis 1980; 24:82-5; PMID:7010567 [PubMed] [Google Scholar]
- 49.Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent) 2005; 18:21-5; PMID:16200144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robbins JB, Myerowitz RL, Whisnant JK, Argaman M, Schneerson R, Handzel ZT, Gotschlich EC. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and III. Infect Immun 1972; 6:651-6; PMID:4118016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldblatt D, Hussain M, Andrews N, Ashton L, Virta C, Melegaro A, Pebody R, George R, Soininen A, Edmunds J. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis 2005; 192:387-93; PMID:15995951; http://dx.doi.org/ 10.1086/431524 [DOI] [PubMed] [Google Scholar]
- 52.Kim HK, Kim H-Y, Schneewind O, Missiakas D. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J 2011; 25:3605-12; PMID:21753082; http://dx.doi.org/ 10.1096/fj.11-187963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol 2005; 3:948-58; PMID:16322743; http://dx.doi.org/ 10.1038/nrmicro1289 [DOI] [PubMed] [Google Scholar]
- 54.Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:1179-86; PMID:22354924; http://dx.doi.org/ 10.1093/cid/cis033 [DOI] [PubMed] [Google Scholar]
- 55.VandenBergh MFQ, Yzerman EPF, van Belkum A, Boelens HAM, Sijmons M, Verbrugh HA. Follow-up of Staphylococcus aureus nasal carriage after 8 Years: Redefining the persistent carrier state. J Clin Microbiol 1999; 37:3133-40; PMID:10488166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Belkum A, Eriksen NHR, Sijmons M, Van Leeuwen W, Van Den Bergh M, Kluytmans J, Espersen F, Verbrugh H. Coagulase and protein A polymorphisms do not contribute to persistence of nasal colonisation by Staphylococcus aureus. J Med Microbiol 1997; 46:222-32; PMID:9126823; http://dx.doi.org/ 10.1099/00222615-46-3-222 [DOI] [PubMed] [Google Scholar]
- 57.Wright AKA, Ferreira DM, Gritzfeld JF, Wright AD, Armitage K, Jambo KC, Bate E, El Batrawy S, Collins A, Gordon SB. Human nasal challenge with Streptococcus pneumoniae is immunising in the absence of carriage. PLoS Pathog 2012; 8:e1002622; PMID:22496648; http://dx.doi.org/ 10.1371/journal.ppat.1002622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNally LM, Jeena PM, Gajee K, Sturm AW, Tomkins AM, Coovadia HM, Goldblatt D. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1–infected South African children. J Infect Dis 2006; 194:385-90; PMID:16826488; http://dx.doi.org/ 10.1086/505076 [DOI] [PubMed] [Google Scholar]
- 59.Madhi SA, Adrian P, Kuwanda L, Cutland C, Albrich WC, Klugman KP. Long-term effect of pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae—and associated interactions with Staphylococcus aureus and Haemophilus influenzae colonization—in HIV-infected and HIV-uninfected children. J Infect Dis 2007; 196:1662-6; PMID:18008250; http://dx.doi.org/ 10.1086/522164 [DOI] [PubMed] [Google Scholar]
- 60.Polack FP, Flayhart DC, Zahurak ML, Dick JD, Willoughby RE. Colonization by Streptococcus pneumoniae in human immunodeficiency virus-infected children. Pediatr Infect Dis J 2000; 19:608-12. [DOI] [PubMed] [Google Scholar]
- 61.Cardoso VC, Cervi MC, Cintra OA, Salathiel AS, Gomes AC. Nasopharyngeal colonization with Streptococcus pneumoniae in children infected with human immunodeficiency virus. J Pediatr 2006; 82:51-7; PMID:16532148 [DOI] [PubMed] [Google Scholar]
- 62.Wolter N, Tempia S, Cohen C, Madhi SA, Venter M, Moyes J, Walaza S, Malope-Kgokong B, Groome M, du Plessis M, et al.. High Nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis 2014; 210:1649-57; PMID:24907383; http://dx.doi.org/ 10.1093/infdis/jiu326 [DOI] [PubMed] [Google Scholar]
- 63.Glennie SJ, Banda D, Gould K, Hinds J, Kamngona A, Everett DDB, Williams NA, Heyderman RS. Defective pneumococcal-specific Th1 responses in HIV-infected adults precedes a loss of control of pneumococcal colonization. Clin Infect Dis 2013; 56:291-9; PMID:23024291; http://dx.doi.org/ 10.1093/cid/cis842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madhi SA, Izu A, Nunes MC, Violari A, Cotton MF, Jean-Philippe P, Klugman KP, von Gottberg A, van Niekerk N, Adrian PV. Longitudinal study on Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus nasopharyngeal colonization in HIV-infected and -uninfected infants vaccinated with pneumococcal conjugate vaccine. Vaccine 2015; 33:2662-9; PMID:25910923; http://dx.doi.org/ 10.1016/j.vaccine.2015.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sepako E, Glennie SJ, Jambo KC, Mzinza D, Iwajomo OH, Banda D, van Oosterhout JJ, A. Williams N, Gordon SB, Heyderman RS. Incomplete recovery of pneumococcal CD4 T cell immunity after initiation of antiretroviral therapy in HIV-infected Malawian adults. PLoS One 2014; 9:e100640; PMID:24959834; http://dx.doi.org/ 10.1371/journal.pone.0100640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lijek RS, Luque SL, Liu Q, Parker D, Bae T, Weiser JN. Protection from the acquisition of Staphylococcus aureus nasal carriage by cross-reactive antibody to a pneumococcal dehydrogenase. Proc Natl Acad Sci 2012; 109:13823-8; http://dx.doi.org/ 10.1073/pnas.1208075109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebon A, Verkaik NJ, Labout JA, de Vogel CP, Hooijkaas H, Verbrugh HA, van Wamel WJ, Jaddoe VW, Hofman A, Hermans PW. Natural antibodies against several pneumococcal virulence proteins in children during the pre-pneumococcal-vaccine era: the generation R study. Infect Immun 2011; 79:1680-7; PMID:21282409; http://dx.doi.org/ 10.1128/IAI.01379-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prevaes SM, van Wamel WJ, de Vogel CP, Veenhoven RH, van Gils EJ, van Belkum A, Sanders EA, Bogaert D. Nasopharyngeal colonization elicits antibody responses to staphylococcal and pneumococcal proteins that are not associated with a reduced risk of subsequent carriage. Infect Immun 2012:IAI 00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barocchi M, Ries J, Zogaj X, Hemsley C, Albiger B, Kanth A, Dahlberg S, Fernebro J, Moschioni M, Masignani V. A pneumococcal pilus influences virulence and host inflammatory responses. Proc Natl Acad Sci U S A 2006; 103:2857-62; PMID:16481624; http://dx.doi.org/ 10.1073/pnas.0511017103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandlik A, Swierczynski A, Das A, Ton-That H. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol 2008; 16:33-40; PMID:18083568; http://dx.doi.org/ 10.1016/j.tim.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basset A, Trzcinski K, Hermos C, O'Brien KL, Reid R, Santosham M, McAdam AJ, Lipsitch M, Malley R. Association of the pneumococcal pilus with certain capsular serotypes but not with increased virulence. J Clin Microbiol 2007; 45:1684-9; PMID:17392439; http://dx.doi.org/ 10.1128/JCM.00265-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Regev-Yochay G, Hanage WP, Trzcinski K, Rifas-Shiman SL, Lee G, Bessolo A, Huang SS, Pelton SI, McAdam AJ, Finkelstein JA. Re-emergence of the type 1 pilus among Streptococcus pneumoniae isolates in Massachusetts, USA. Vaccine 2010; 28:4842-6; PMID:20434550; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kulohoma BW, Gray K, Kamng'ona A, Cornick J, Bentley SD, Heyderman RS, Everett DB. Piliation of invasive Streptococcus pneumoniae isolates in the era before pneumococcal conjugate vaccine introduction in Malawi. Clin Vaccine Immunol 2013; 20:1729-35; PMID:24027261; http://dx.doi.org/ 10.1128/CVI.00403-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feder G, Regev U. Biological interactions and environmental effects in the economics of pest control. J Envir Econom Manag 1975; 2:75-91; http://dx.doi.org/ 10.1016/0095-0696(75)90001-7 [DOI] [Google Scholar]
- 75.Creech CBI, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J 2005; 24:617-21; PMID:15999003; http://dx.doi.org/ 10.1097/01.inf.0000168746.62226.a4 [DOI] [PubMed] [Google Scholar]
- 76.Spijkerman J, Prevaes SM, van Gils EJ, Veenhoven RH, Bruin JP, Bogaert D, Wijmenga-Monsuur AJ, van den Dobbelsteen GP, Sanders EA. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One 2012; 7:e39730; PMID:22761879; http://dx.doi.org/ 10.1371/journal.pone.0039730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho P-L, Chiu SS, Chan MY, Gan Y, Chow K-H, Lai EL, Lau Y-L. Molecular epidemiology and nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus among young children attending day care centers and kindergartens in Hong Kong. J Infect 2012; 64:500-6; PMID:22406412; http://dx.doi.org/ 10.1016/j.jinf.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 78.van Gils EJ, Hak E, Veenhoven RH, Rodenburg GD, Bogaert D, Bruin JP, van Alphen L, Sanders EA. Effect of seven-valent pneumococcal conjugate vaccine on Staphylococcus aureus colonisation in a randomised controlled trial. PLoS One 2011; 6:e20229; PMID:21695210; http://dx.doi.org/ 10.1371/journal.pone.0020229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biesbroek G, Wang X, Keijser BJF, Eijkemans RMJ, Trzciński K, Rots NY, Veenhoven RH, Sanders EAM, Bogaert D. Seven-valent pneumococcal conjugate vaccine and nasopharyngeal microbiota in healthy children. Emerg Infect Dis 2014; 20:201-10; PMID:24447437; http://dx.doi.org/ 10.3201/eid2002.131220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siegrist C-A. Neonatal and early life vaccinology. Vaccine 2001; 19:3331-46; PMID:11348697; http://dx.doi.org/ 10.1016/S0264-410X(01)00028-7 [DOI] [PubMed] [Google Scholar]
- 81.Chibuk TK, Robinson JL, Hartfield DS. Pediatric complicated pneumonia and pneumococcal serotype replacement: Trends in hospitalized children pre and post introduction of routine vaccination with pneumococcal conjugate vaccine (PCV7). Eur J Pediatr 2010; 169:1123-8; PMID:20383524; http://dx.doi.org/ 10.1007/s00431-010-1195-6 [DOI] [PubMed] [Google Scholar]
- 82.Cohen R, Levy C, Thollot F, de La Rocque F, Koskas M, Bonnet E, Fritzell B, Varon E. Pneumococcal conjugate vaccine does not influence Staphylococcus aureus carriage in young children with acute otitis media. Clin Infect Dis 2007; 45:1583-7; PMID:18190319; http://dx.doi.org/ 10.1086/523734 [DOI] [PubMed] [Google Scholar]
- 83.Dunne EM, Manning J, Russell FM, Robins-Browne RM, Mulholland EK, Satzke C. The effect of pneumococcal vaccination on nasopharyngeal carriage of S. pneumoniae, H. influenzae, M. catarrhalis, and S. aureus in Fijian children. J Clin Microbiol 2011:JCM 06589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Revai K, Mamidi D, Chonmaitree T. Association of nasophyaryngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis 2008; 46:e34-e7; PMID:18205533; http://dx.doi.org/ 10.1086/525856 [DOI] [PMC free article] [PubMed] [Google Scholar]


