Figure 1.
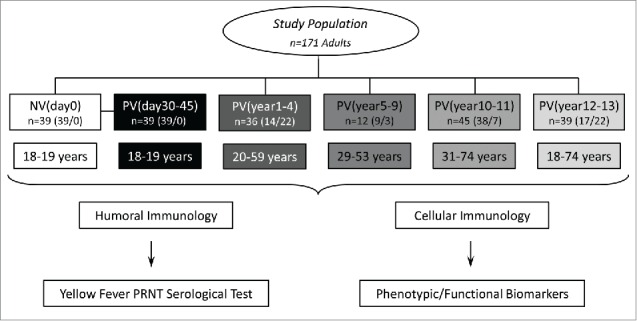
Flowchart illustrating the study population and experimental design. The study was based on two branches: The humoral (A) and cellular (B) immunology analysis. The eligible population comprises 171 adults. Blood collections (without anticoagulant for humoral analysis and in heparinized tubes for cellular immunology assessment) were performed prior vaccination (NVday0 (n=39) and at different timepoints after primary vaccination: PVday30-45 (n=39); PVyear1-4 (n=36); PVyear5-9 (n=12); PVyear10-11 (n=45) and PVyear12-13 (n=39).
