Abstract
Similar to estrogens, bone morphogenetic protein 4 (BMP4) promotes the accumulation of more metabolically active subcutaneous fat and reduction of visceral fat. However, whether there is a cross-talk between BMP4 and estrogen signaling remained unknown. Herein, we found that BMP4 deficiency in white adipose tissue (WAT) increased the estrogen receptor α (ERα) level and its signaling, which prevented adult female mice from developing high fat diet (HFD)-induced obesity and insulin resistance; estrogens depletion up regulated BMP4 expression to overcome overt adiposity and impaired insulin sensitivity with aging, and failure of BMP4 regulation due to genetic knockout led to more fat gain in aged female mice. This mutual regulation between BMP4 and estrogen/ERα signaling may also happen in adipose tissue of women, since the BMP4 level significantly increased after menopause, and was inversely correlated with body mass index (BMI). These findings suggest a counterbalance between BMP4 and estrogen/ERα signaling in the regulation of adiposity and relative metabolism in females.
Keywords: BMP4, Estrogen, ERα, White adipose tissue, Glucose metabolism
Highlights
-
•
BMP4 knockout improves ERα stability and its signaling in WAT of female mice.
-
•
BMP4 knockout female mice get higher energy expenditure.
-
•
Depletion of estrogens up regulates BMP4 level in adipose tissue.
-
•
Failure of BMP4 regulation leads to obesity and insulin resistance in aged females.
Estrogens play a beneficial role in regulating adiposity and glucose metabolism, however not all women with low estrogen levels become obese and develop insulin resistance. Such variation may be related to other factors involved. In the present study, we revealed a reciprocal interaction between bone morphogenetic protein 4 (BMP4) and estrogen/ERα signaling in regulating fat accumulation and insulin sensitivity. Our findings may explain why cycling females are less prone to gain weight and less susceptible to glucose dysmetabolism than menopausal females and males. The regulation of BMP4 may offer a new opportunity of intervention in the control of excessive obesity.
1. Introduction
Adipose tissue is a metabolically dynamic organ for the storage of excess energy, and it also serves as an endocrine organ capable of synthesizing hormones that regulate metabolic homeostasis (Scherer, 2006). Adipose tissue buffers energy by hyperplasia and/or hypertrophy of adipocytes when increased energy storage is needed. However, overt adiposity results in obesity. The obese condition is a risk factor for the development of many disorders, particularly insulin resistance and diabetes (Smyth and Heron, 2006). Numerous factors, including genetics, dietary metabolites, hormones, or a variety of secretary cytokines have been documented to regulated the adiposity and glucose metabolism, how these factors affect adipocytes and the relative metabolism are largely unknown.
Estrogens are known to regulate fat accumulation, energy balance, and insulin sensitivity (Chen et al., 2009, Mauvais-Jarvis et al., 2013). Being abundantly produced in premenopausal women, these hormones promote and maintain the typical female-type of fat distribution characterized by accumulation of adipose tissue in the subcutaneous fat depot with only modest accumulation of intra-abdominal adipose tissue (Elbers et al., 1999, Enzi et al., 1986, Shi and Clegg, 2009, Toth et al., 2001), suggesting that premenopausal women are less prone to developing insulin resistance than men of the same age (Geer and Shen, 2009, Vistisen et al., 2014). The role of estrogens in adiposity is evidenced by the fact that body weight increases under low estrogen conditions such as menopause, ovariectomy, or the lack of a functional aromatase gene, and can be corrected by 17β-estradiol treatment (Gambineri et al., 2002, Jones et al., 2000, Misso et al., 2003, Takeda et al., 2003). Similarly, the incidence of obesity and insulin resistance in women gradually increases after menopause (Yang et al., 2010). The regulation of adiposity and improved glucose metabolism caused by estrogens in adipose tissue occurs through an ERα-mediated response (Barros and Gustafsson, 2011, Heine et al., 2000, Ohlsson et al., 2000). ERα deficient mice exhibit increased adipose tissue mass in the absence of differences in energy intake. Some researchers reported that polymorphisms in the ERα gene are associated with unhealthy upper-body obesity in middle-aged women (Deng et al., 2000, Fox et al., 2005, Okura et al., 2003). Estrogens play a beneficial role in regulating adiposity and glucose metabolism, however not all women with low estrogen levels become obese and develop insulin resistance. Such variation may be related to the heterogeneity of host genotype.
Bone morphogenetic protein 4 (BMP4) is one of the morphogens involved in vertebrate embryonic patterning and evolution of mesoderm tissues (Bier and De Robertis, 2015, Hogan, 1996). Genetic variants of BMP4 were associated with triglyceride levels and high-density lipoprotein cholesterol, suggesting its role in metabolism (Tang et al., 2013). Our previous works demonstrate that BMP4 participates in adipose tissue development and metabolism (Huang et al., 2009, Qian et al., 2013). BMP4 induces brown fat-like changes in white adipose tissue as well as increased insulin sensitivity in mice. Moreover, high level of BMP4 in adipose tissue leads to both accumulation of more metabolically active subcutaneous adipocytes, and the reduction in the size of the visceral fat mass-an effect similar to that induced by estrogens. Knockout of BMP4 in adipose tissue results in increased size of adipocytes and fat mass in both subcutaneous and visceral fat of adult male mice, a phenomenon not observed in female mice. These results indicate that cross-talks between BMP4 and estrogen signaling may exist in females to balance fat mass and metabolism.
Herein, we investigated whether BMP4 cross-talks with estrogen and ERα signaling to regulate adiposity and glucose metabolism. We found that BMP4 and estrogen/ERα were negatively regulated by each other: the inactivation of one pathway leads to the activation of the other pathway that can prevent mice from overt adiposity and metabolic disorder. In women, BMP4 levels in subcutaneous WAT significantly increased after menopause, and inversely correlated with BMI in postmenopausal stage. These findings support a counterbalance between BMP4 and ERα signaling in the regulation of adiposity and glucose metabolism in females.
2. Materials & Methods
2.1. Human Adipose Tissue Samples
Subcutaneous adipose tissues were obtained from patients underwent surgery irrelevant to metabolic disease in Shanghai Jiaotong University Affiliated Sixth and Ninth People's Hospital and Obstetrics and Gynecology Hospital of Fudan University. This study was approved by the ethics committees of Fudan University Institutes of Biomedical Sciences and was in accordance with the principle of the Helsinki Declaration II. The written informed consent was obtained from each participant.
2.2. Generation of Adipose Tissue-specific BMP4 Knockout Mice
To generate mice with an adipocyte-Specific knockout of BMP4, Bmp4LoxP/LoxP mice (generously provided by Brigid Hogan, Department of Cell Biology, Duke University Medical Center, Durham, NC) were crossed with mice expressing Cre recombinase under the control of the adipocyte-specific promoter Fabp4 (Jackson Laboratory). Genotyping was performed by PCR. Studies were performed in Fabp4-Cre-Bmp4LoxP/LoxP mice and littermate controls lacking the LoxP sites (Fabp4-Cre-Bmp4 +/+). Mice were maintained under 12-h light/12-h dark cycles with unlimited access to food and water. Mice were fed with chow or an HFD (51% kcal in fat, beginning at age 6 wk). Body weight was monitored every week until euthanization and tissue collection. The data shown were collected from experiments on male and female mice. All studies were approved by the Animal Care and Use Committee of the Fudan University Shanghai Medical College and followed the National Institute of Health guidelines on the care and use of animals.
2.3. Isolation of Preadipocytes and Adipocytes From Adipose Tissue
Adipose tissue was harvested and the SVF cells were isolated by collagenase digestion (collagenase VIII; Sigma). The digested tissue was filtered through a 100-μm mesh filter to remove debris and was centrifuged. The adipocytes floated above the supernatant. The preadipocyte-containing pellet was suspended with an ammonium chloride lysis buffer to remove red blood cells.
2.4. Cell Culture
Primary preadipocytes were cultured in DMEM/F12 containing 10% FBS. Multipotent stem cell line C3H10T1/2 were in DMEM containing 10% FBS. Adipocyte differentiation was induced in preadipocytes cultures by treating confluent cells for 48 h in medium containing 10% FBS, 0.5 mM isobutylmethylxanthine, 125 nM indomethacin, 1 μM dexamethasone, 850 nM insulin, 1 nM T3, and 1 μM rosiglitazone. 2 days after induction, cells were switched to maintenance medium containing 10% FBS, 850 nM insulin, 1 nM T3, and 1 μM rosiglitazone and maintained for 2 days. At day 5, the medium was replaced with DMEM (phenol free) containing 10% charcoal stripped FBS (Gibco), and the cells were grown in such a medium for another 2 days. After 6 days of differentiation induction, cells were incubated with or without 17β-estradiol (Sigma-Aldrich)for the indicated time periods. 17β-estradiol was dissolved in 0.1% ethanol and sterile medium.
2.5. Ovariectomy
At 2 month of age, female mice were matched for body composition and underwent sham operation or bilateral OVX. Anesthesia was performed with an i.p. injection of chloral hydrate (0.04 ml/10 g of body weight). Mice were bilaterally ovariectomized through a single dorsal midline incision across the lumbar region, making both ovaries accessible. The ovary-attached fat pad was gently grasped to lift and exteriorize the ovary. Subsequently, the periovarian sac was peeled back over the surface of the ovary, allowing removal of the whole ovary. Both ovaries of the ovariectomized group mice were removed, whereas ovaries of the sham-operated group were left in situ.
2.6. Glucose and Insulin Tolerance Tests
To test glucose tolerance, mice were injected i.p. with d-glucose (2 mg/g body weight) after an overnight fast, and tail blood glucose levels were monitored. To test insulin tolerance, mice fed ad libitum were injected i.p. with human insulin (Eli Lilly) (0.75 mU/g body weight) around 2:00 PM, and tail blood glucose levels were monitored.
2.7. Serum Analyses
Mice blood was collected by retroorbital bleeding at different ages. Sera were then prepared and used for measurements. Serum levels of 17-estradiol were determined using an ELISA kit (Cayman Chemical, Ann Arbor, MI).
2.8. H&E Staining and Cell Size Quantitation
Standard H&E staining was performed on 5-μm paraffin sections of WAT and interscapular brown adipose tissue. Cell diameter was measured in the H&E-stained sections of three individual samples in each group using Image J.
2.9. Antibodies and Immunoblotting
To determine the protein levels of BMP4 and ERα, adipose tissue or cultured cells were homogenized in lysis buffer containing 2% (wt/vol) SDS and 60 mM Tris·HCl (pH 6.8) and were subjected to electrophoresis. Proteins then were transferred onto PVDF membrane and immunoblotted with specific antibodies. The anti-BMP4 antibody was from Millipore (catalog number: MAB 1094; Dilution: 1:2000), anti-ERα was from Santa Cruz Biotechnology (catalog number: SC-787; Dilution: 1:500), and anti-HSP90 was also from Santa Cruz Biotechnology (catalog number: SC-7947; Dilution: 1:1000).
2.10. Real-time quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). PrimeScript RT Master Mix (TaKaRa) was used for first strand cDNA synthesis with random primers. Real-time quantitative PCRs were carried out with 2 × PCR Master Mix (Power SYBR Green; Applied Biosystems, Foster City, CA) on an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems). The primers were from (PrimerBank http://pga.mgh.harvard.edu/primerbank/), and listed in Supplementary Table 1.
2.11. Statistical Analyses
All results are presented as means ± SEM. A 2-way, 2-tailed t-test was used for comparisons. A difference was considered significant at *p < 0.05, **p < 0.01, and ***p < 0.001.
3. Results
3.1. Female Mice With BMP4 Knockout in Adipose Tissue Are Protected From HFD Induced Obesity and Insulin Resistance
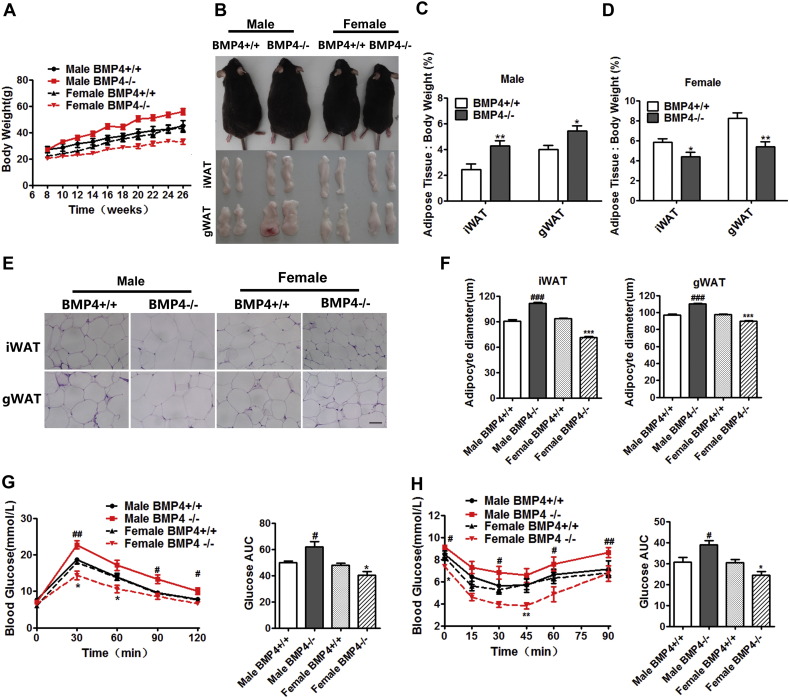
Our previous study showed that BMP4 knockout in adipose tissue caused male mice to gain more body weight and fat mass (Qian et al., 2013). By contrast, female BMP4 knockout mice did not gain more body weight (Supplementary Fig. 1A, B) or fat mass (Supplementary Fig. 1C) than wild type (WT) mice fed a normal chow (NC). To determine whether the regulation of adipose tissue is abnormal in female BMP4 knockout mice, animals were subjected to a high fat diet (HFD) from age 6 to 26 weeks. Male BMP4 knockout mice gained more body weight (Fig. 1A) and exhibited a higher fat mass index (fat weight relative to body weight) for both inguinal and gonadal WAT than female BMP4 knockout mice (Fig. 1A–D). The increased fat mass in male BMP4 knockout mice correlated with larger size of adipocytes and more lipid accumulation within adipocytes (Fig. 1E, F). Notably, even with the challenge of a HFD, female BMP4 knockout mice gained less weight and had a lower fat mass index for both gonadal and inguinal WAT than matched WT control mice (Fig. 1A–D). Adipocytes in WAT of BMP4 knockout female mice were smaller than those of WT mice (Fig. 1E, F). There was no marked difference in the appearance (Supplementary Fig. 1D) and fat index (Supplementary Fig. 1D) of BAT between female BMP4 knockout mice and control mice. We concluded that female mice with disrupted expression of BMP4 in adipose tissue were protected from weight gain and fat accumulation upon HFD challenge.
Supplementary Fig. 1.
Phenotype of BMP4 knockout (BMP4 −/−) and control (BMP4 +/+) mice of different gender. (A) Growth curve of BMP4 knockout (BMP4 −/−) and control (BMP4 +/+) mice from 8 to 24 weeks. n = 8. (B) Comparison of BMP4 knockout and control mice of different gender. (C) Comparison of adipose tissue from BMP4 knockout and control mice of different gender. (D) Hematoxylin and eosin staining of liver from BMP4 knockout and control mice on HFD. Scale bar: 25 μm. (E) Fat index (percentage of fat pad weight to the whole body weight) of BAT in BMP4 knockout and control male (C) and female (D) mice. n = 8. Data were collected from mice on normal chow diet and expressed as means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 1.
Adiposity and glucose metabolism of BMP4 knockout mice. (A) Growth curve of BMP4 knockout and wild type control mice on HFD from 8 to 26 weeks. n = 6. (B) Representative pictures of mice and fat pads of BMP4 knockout (left panel) and control mice (right panel). (C, D) Fat index (percentage of fat pad weight to the whole body weight) of inguinal WAT and gonadal WAT in BMP4 knockout and control male (C) and female (D) mice. n = 8. (E) H&E staining of inguinal WAT and gonadal WAT from BMP4 knockout and control mice. Scale bar: 25 μm. (F) Quantification of adipocyte diameter of inguinal WAT, gonadal WAT and BAT from BMP4 knockout and control mice. (Data were collected using Image J software from H&E staining section of 3 individual mice, 5 fields per mouse, 10–15 cells per field in each group). (G) Glucose concentrations during an intraperitoneal glucose tolerance test (I) (n = 8) and quantification of AUG (area under curve) from BMP4 knockout and control mice. n = 6–9. (H) Glucose concentrations during an intraperitoneal insulin tolerance test (n = 7) and quantification of AUG from BMP4 knockout and control mice. n = 6–10. BMP4 +/+: wild type control; BMP4 −/−: BMP4 knockout. Data are collected from mice on HFD at age of 6 months except indicated, and expressed as means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
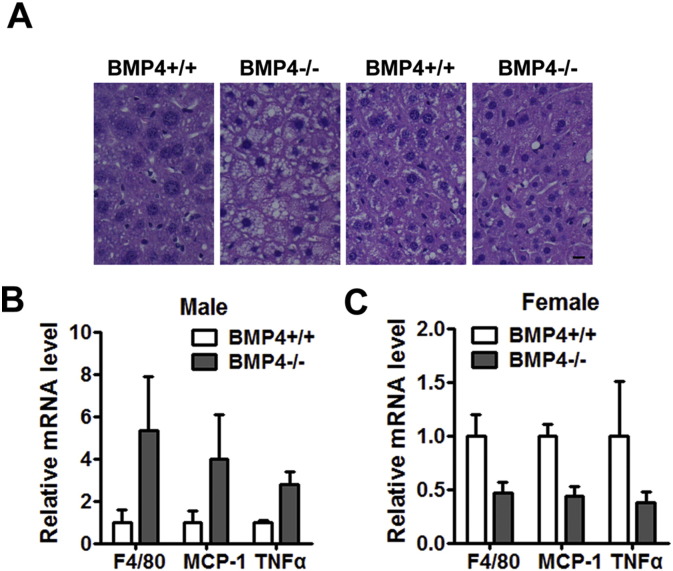
Given that overt fat accumulation increases the risk for insulin resistance and type 2 diabetes in both humans and mice (Smyth and Heron, 2006), we investigated the ability of glucose disposal in mice by performing glucose tolerance tests (GTT) and insulin tolerance tests (ITT). When challenged with an intraperitoneal glucose or insulin load, female BMP4 knockout mice suffered less impairment of glucose or insulin intolerance than WT control mice, whereas the male BMP4 knockout mice became more glucose and insulin tolerant (Fig. 1G, H). Female BMP4 knockout mice were also protected from fatty liver as shown by H&E staining of liver slides (Supplementary Fig. 2A), and from inflammation in adipose tissue as indicated by the expression of relative genes (Supplementary Fig. 2B). These results suggested that effects of BMP4 on WAT related to adiposity and glucose metabolism is gender dependent.
Supplementary Fig. 2.
Disruption of BMP4 expression prevent female mice from fatty liver and inflammation in adipose tissue. (A) Hematoxylin and eosin staining of liver from BMP4 knockout and control mice on HFD. Scale bar: 25 μm. (B) RT-qPCR analysis of adipose tissue for expression of inflammatory factors in visceral WATfromBMP4 knockout and control male mice. n = 4. (C) RT-qPCR analysis of adipose tissue for expression of inflammatory factors in visceral WAT fromBMP4 knockout and control male mice. n = 4.
3.2. ERα Signaling Is Enhanced in BMP4 Knockout WAT of Female Mice
Because estrogens and activation of ERα play a predominant role in regulating adiposity and insulin sensitivity, the observed dimorphism between male and female mice may involve differential estrogens and/or ERα signaling. The level of serum estradiol and ERα in WAT in six months old mice were examined with enzyme-linked immunosorbent assay (Elisa) and Western blot, respectively. Down-regulation of BMP4 expression in adipose tissue by genetic knockout had no effect on serum levels of 17β-estradiol (Fig. 2A). The mRNA level of ERα was unchanged in female BMP4 knockout mice (Fig. 2B). However, the protein level of ERα (Fig. 2C) increased in both inguinal and gonadal WAT in female BMP4 knockout mice as shown by Western blot. The reported targets of ERα - glutathione peroxidase 3 (GPX3), Liver X receptor α and β (LXRα and LXRβ) were also determined. In inguinal WAT, GPX3 was elevated in female knockout mice, whereas LXRα and LXRβ were not changed. In gonadal WAT, all of the three targets were elevated (Fig. 2D). Unlike the results found for females, BMP4 knockout in male adipose tissue led to lower levels of ERα (Supplementary Fig. 3A), indicating that effect of BMP4 on ERα may depend on the level of estrogens (Supplementary Fig. 3B).
Fig. 2.
Enhanced ERα signaling in WAT of BMP4 knockout female mice. (A) Serum 17β-estradiol level showed by Elisa in BMP4 knockout and control mice. n = 4. (B) RT-qPCR analysis of WAT for expression of ERα from female BMP4 knockout and control mice on HFD at age of 6 months. n = 5. (C) Representative Western blotting analysis of ERα expression level in adipose tissue from BMP4 knockout and control female mice on HFD at age of 6 months. Relative grey intensity of the band was quantitated using Image J software. n = 6–7. (D) RT-qPCR analysis of WAT for expression of ERα target genes GPX3, LXRα and LXRβ from 6 months old mice on HFD. n = 6. (E) Daily food intake of control and knockout female mice maintained on a HFD, 8 mice per group, weigh each week for 5 weeks. (F) Whole-body oxygen consumption rate (VO2) of control and knockout female mice of 6 months during a 12-hour dark/12-hour light cycle measured in metabolic cage, n = 8. (G) RT-qPCR analysis of WAT for expression of genes of β oxidation from female BMP4 knockout and control mice on HFD at age of 6 months, n = 8. Data are expressed as means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. BMP4 +/+: wild type control; BMP4 −/−: BMP4 knockout.
Supplementary Fig. 3.
BMP4 regulation of ERα depends on levels of estrogens. (A) Western blotting analysis of ERα expression level in adipose tissue from male BMP4 knockout and control mice on HFD at age of 6 months. Relative grey intensity of the band was quantified using Image J software. n = 6. (B) Multipotent C3H10T1/2 cells stably expressing ERα-Flag were induced to differentiation and treated with 20 ng/ml BMP4 in presence of estradiol at different concentration for 12 h, and the protein level of ERα was examined with anti-Flag antibody by Western blot. Relative grey intensity of the band from 4 individual experiments was quantified using Image J software.
Regarding that ERα activity reduces fat accumulation, the leanness of female BMP4 knockout mice may be attributed to the elevation of ERα signaling. To determine whether the elevated ERα signaling altered energy balance, we calculated the energy intake and expenditure. There was no significant difference in food intake between female knockout and control mice (Fig. 2E). Oxygen consumption rates of BMP4 knockout mice were substantially increased relative to those of control mice (Fig. 2F). Consistent with this elevated energy expenditure, expression of genes for β oxidation-Acox1 (acyl-Coenzyme A oxidase 1), VLCAD (acyl-Coenzyme A dehydrogenase, very long chain), MCAD (acyl-Coenzyme A dehydrogenase, medium chain), and CPT1b (carnitine palmitoyltransferase 1b) - in both inguinal and gonadal WAT increased substantially in the BMP4 knockout mice compared to those in control (Fig. 3G).
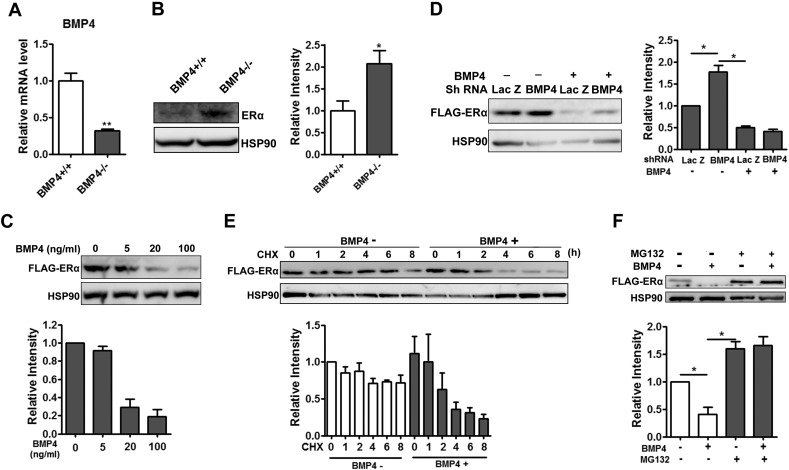
Fig. 3.
BMP4 knockout Increased protein stability of ERα in adipocytes. (A) Preadipocytes isolated from adipose tissue of female BMP4 knockout and control mice were stimulated to adipogenesis. Differentiated adipocytes were maintained for more 12 h in presence of 100 nM estradiol, and RT-qPCR analysis of BMP4 expression level. (B) Western blotting analysis of ERα expression level in differentiated adipocyte indicated in A. (C) Differentiated multipotent C3H10T1/2 cells stably expressing FLAG tagged ERα were treated with BMP4 at different concentration in presence of 100 nM estradiol for 12 h, and the level of ERα was examined with anti-Flag antibody by Western blot. (D) Multipotent C3H10T1/2 cells stably expressing ERα-FLAG were induced to differentiation and at day 4 and cells were infected with adenovirus expressing BMP4 shRNA and control Lac Z sh, the of ERα was examined with anti-Flag antibody by Western blot 48 h after infection. (E) Differentiated adipocytes from C3H10T1/2 cells stably expressing ERα-FLAG treated with or without of BMP4 were incubated with cycloheximide (20 μM) for the indicated times and ERα was examined by Western blot. (F) Differentiated C3H10T1/2 cells pretreated with cycloheximide (20 μM) and incubated with combination of 20 ng/ml BMP4 and 25 μM MG132 for 8 h. ERα was examined by Western blot. For Western blot, relative grey intensity of the band from three individual experiments was quantitated using Image J software.
3.3. BMP4 Knockout Increased Protein Stability of ERα in Adipocytes
The BMP4 inhibition of ERα in female mice was further verified in cultured adipocytes. Preadipocytes from inguinal WAT of female WT and BMP4 knockout mice were isolated, cultured and induced to differentiate. About 90% of cells were differentiated into adipocytes. BMP4 knockout (Fig. 3A) was confirmed by real-time PCR. Cultured mature adipocytes were maintained for 12 h more in the medium with 100 nM estradiol, and the level of ERα was determined by Western blot. We found that the amount of ERα protein increased in cultured BMP4 knockout adipocytes (Fig. 3B), consistent with the change in vivo.
To study how BMP4 regulates ERα, multipotent C3H10T1/2 cells stably expressing FLAG-tagged ERα were induced to differentiate. In this cell model, BMP4 treatment decreased ERα level in a dose dependent manner (Fig. 3C), and knockdown of BMP4 with short hairpin RNA increased the level of ERα, an effect that could be reversed by BMP4 treatment (Fig. 3D). Moreover, the regulation of ERα by BMP4 was dependent upon the level of estrogens. Only at high concentrations of estradiol (100 nM), BMP4 showed obvious inhibitory effect on ERα protein level (Supplementary Fig. 3B).
It is reasonable to believe that BMP4 promotes the degradation of ERα protein, since the mRNA level of ERα in BMP4 knockout adipocyte was not much different from that in wild-type adipocyte. The differentiated adipocytes from C3H10T1/2 cells treated with or without of BMP4 were incubated with cycloheximide (20 μM) for different times to examine ERα protein stability. We found that BMP4 treatment significantly decreased the half-life of ERα (Fig. 3E). The inhibition of proteasomal degradation with MG132 (25 μM) completely abolished the BMP4 inhibition of ERα protein (Fig. 3F). These data support the notion that BMP4 regulates ERα signaling mainly through the regulation of the stability of ERα proteins.
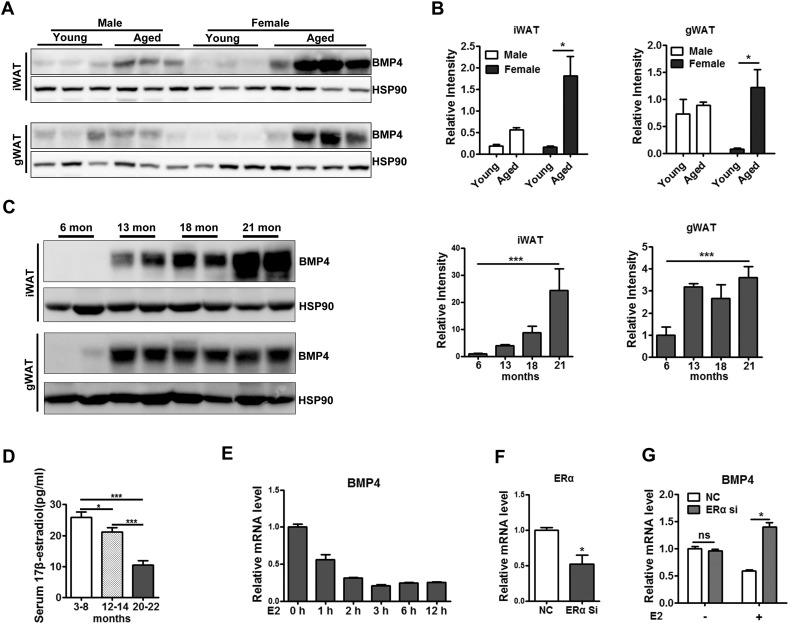
3.4. Decreased Level of Estrogens Up-regulates BMP4 Expression in Adipose Tissue
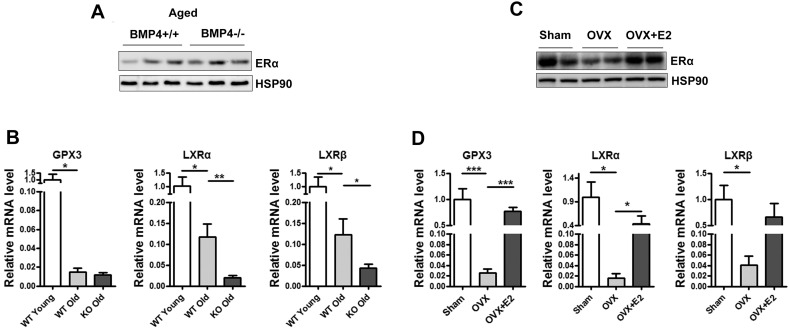
Above results showed that in adult female BMP4 knockout mice with high levels of estrogens, overt adiposity and impaired insulin sensitivity may be prevented by up-regulation of ERα signaling. However, endogenous estrogen levels decrease as female mice age. We found that BMP4 knockout did not obviously increase ERα level in aged mice (Supplementary Fig. 4A), which is consistence with the result that BMP4 regulation of ERα depends on the level of estrogen (Supplementary Fig. 3B). Moreover, we examined the expression of the target genes downstream of ERα and found that the expression of GPX3 decreased by about 80 fold, that of LXRα and LXRβ also decreased by 10 fold as compared with young mice (Supplementary Fig. 4B). The data suggested the estrogen/ERα signaling was inhibited in aged mice, and would not efficiently perform its benefit function on adipose tissue.
Supplementary Fig. 4.
(A): Western blotting analysis of ERα expression level in white adipose tissue of 22 months aged female mice on NC. (B): RT-qPCR analysis of WAT in A for expression of ERα target genes GPX3, LXRα and LXRβ. (C): Western blotting analysis of ERα expression level in white adipose tissue of OVX mice. (D): RT-qPCR analysis of WAT in C for expression of ERα target genes GPX3, LXRα and LXRβ.
To assess the effect of BMP4 knockout in aged female mice, we then examined the regulation of BMP4 in both young and aged mice. WT male and female mice fed NC were examined at age of six months and 22 months of age. Expression of BMP4 in WAT was determined by Western blot. As illustrated in Fig. 4A and B, BMP4 was expressed in both inguinal and gonadal WAT, with higher expression in young male mice than female mice. BMP4 expression in WAT also showed age-related differences (Fig. 4A, B). Aged male mice expressed a higher level of BMP4 than young male mice, but this difference was not significant. However, the increase of BMP4 expression with aging was dramatic in female mice, and the aged female mice at the age of 22 months had much higher level of BMP4 than male mice at the same age. Interestingly, there was an inverse correlation between serum 17β-estradiol (Fig. 4D) and BMP4 levels (Fig. 4C) in WAT from female mice at different ages, suggesting that estrogens may play a role in the regulation of BMP4 expression in WAT during aging. To validate the effect of estrogens on BMP4 in adipocytes, we treated adipocytes differentiated from primary preadipocyte with estradiol (100 ng/ml). We found that the estradiol treatment gradually decreased BMP4 mRNA level, which were then maintained at a relatively low level (Fig. 4E). In contrast, knock down of ERα (Fig. 4F) increased the mRNA level of BMP4 in the presence of estradiol (Fig. 4G). These results indicate that estrogens indeed down-regulates BMP4 expression through ERα signaling.
Fig. 4.
Decreased level of estrogens up regulates BMP4 expression in adipose tissue. (A) Western blotting analysis of ERα expression level in inguinal and gonadal white adipose tissue from young (6 months old) and (22 months old) old female mice. (B) Relative grey intensity of the band showed in A quantitated using Image J software. n = 3–4. (C) Western blotting analysis of BMP4 expression level in inguinal and gonadal white adipose tissue from female mice at indicated ages. Relative grey intensity of the band was quantitated using Image J software. n = 4. (D) Serum 17β-estradiol level showed by Elisa in female mice at indicated ages. n = 4–8. (E) RT-qPCR analysis for expression of BMP4 in differentiated adipocytes from primary isolated preadipocytes treated with estradiol (100 ng/ml). (data from three independent experiments). (F) ERα expression level showed by RT-qPCR in adipocytes with ERα SiRNA transfection (data from three independent experiments). (G) Relative expression level of BMP4 showed by RT-qPCR in differentiated adipocytes (data from three independent experiments). BMP4 +/+: wild type control; BMP4 −/−: BMP4 knockout.
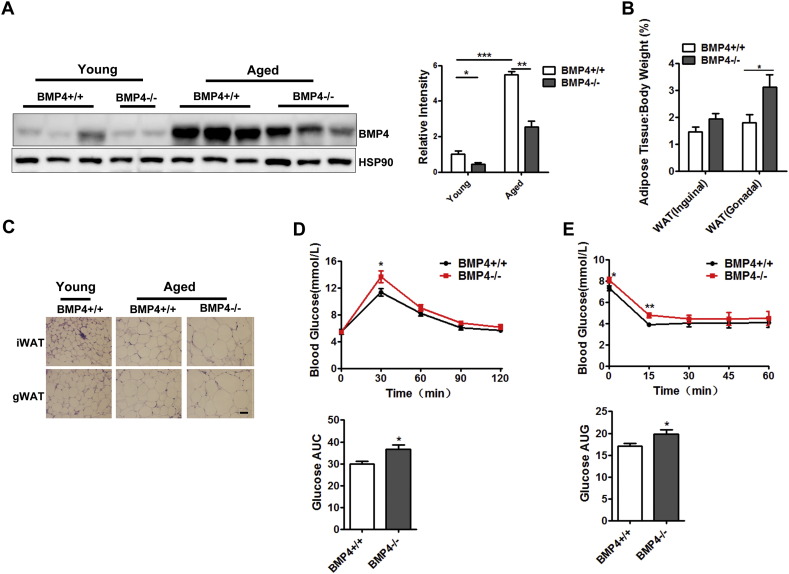
3.5. Aged BMP4-knockout Female Mice Are Prone to Developing Obesity and Insulin Resistance
Talking advantage of the BMP4 knockout mice, in which BMP4 expression cannot be upregulated during aging (Fig. 5A), we examined whether miss-regulation of BMP4 affects the fat accumulation in aged female mice. We found that aged female BMP4 knockout mice (22 months) had a higher fat index with a remarkable change in the amount of gonadal WAT (Fig. 5B). Adipocytes enlarge as mice age, and old BMP4 knockout mice developed even larger adipocytes than WT mice (Fig. 5C). Accordingly, female BMP4 knockout mice displayed more severe insulin resistance than WT mice as shown by results of GTT (Fig. 5D) and ITT (Fig. 5E).
Fig. 5.
Aged BMP4-knockout female mice are prone to developing obesity and insulin resistance. (A) Western blotting analysis of BMP4 expression level in gonadal white adipose tissue from BMP4 knockout and control mice at different ages. Relative grey intensity of the band was quantitated using Image J software. n = 6. (B) Fat index of inguinal WAT and gonadal WAT in aged BMP4 knockout and control mice. n = 6–7. (C) H&E staining of inguinal WAT and gonadal WAT from BMP4 knockout and control mice. Scale bar: 20 μm. (D) Glucose concentrations during an intraperitoneal glucose tolerance test and quantification of AUG (area under curve) from aged BMP4 knockout and control mice. n = 7. (E) Glucose concentrations during an intraperitoneal insulin tolerance test and quantification of AUG from aged BMP4 knockout and control mice. n = 6. Data are collected from mice on NC at age different ages (Young: 6 months; Aged 22 months), and expressed as means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
3.6. Ovariectomized BMP4-knockout Females Are Prone to Developing Obesity and Insulin Resistance, Which Can Be Reduced by Estradiol Treatment
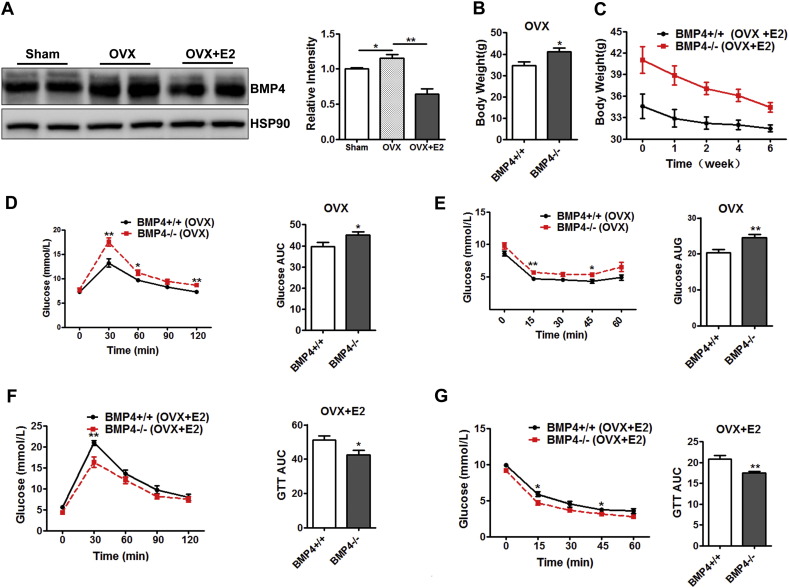
To further verify the role of estrogens in the regulation of BMP4 expression and its consequence effects on adipose tissue, female WT and KO mice (2 months old) were ovariectomized (OVX) and maintained for eight weeks to deplete ovarian steroid hormones, followed by intraperitoneal administration of estradiol (E2) for six weeks. The level of ERα in white adipose tissue was firstly examined and the result of Western blot showed that ERα in OVX mice decreased as compared to sham mice, which was restored after E2 administration (Supplementary Fig. 4C). The estrogen/ERα signaling was also inhibited in OVX mice with estrogens depleted as shown by the expression of target genes (GPX3, LXRα and LXRβ) (Supplementary Fig. 4D).
Similar to the results found in aged mice, the removal of endogenous estrogens led to elevated levels of BMP4 in WAT, an effect that could be reversed by estradiol treatment (Fig. 6A). After OVX, female BMP4 knockout mice gained more body weight than WT mice (Fig. 6B), and E2 treatment decreased body weight in both WT and knockout mice (Fig. 6C). Although E2 treatment for 6 weeks did not reverse the body weight of BMP4 knockout mice to a level lowered than WT mice (The phenotype of adult mice with normal level of estrogens), the difference between them diminished (Fig. 6C). Female OVX BMP4 knockout mice also showed more sever impairments in insulin sensitivity than WT mice as shown by the results of GTT (Fig. 6D) and ITT (Fig. 6E). This impairment was also reversed by administration of exogenous estrogen E2 (Fig. 6F, G). Taken together, elevated BMP4 expression appears to compensate the lack of endogenous estrogens to overcome the defect in adipose tissue under the condition of low endogenous estrogens, and the failure of BMP4 response may lead to defects in adipose tissue and relative glucose metabolism.
Fig. 6.
Estradiol reduced obesity and insulin resistance of ovariectomized female BMP4-knockout mice. WT and KO female mice at age of 2 months were ovariectomized and maintained for 8 weeks to deplete ovarian steroid hormone, followed by ip administration of estradiol for 6 weeks. (A) Western blotting analysis of BMP4 expression level in WAT. Relative grey intensity of the band was quantitated using Image J software. n = 4. (B) Body weight of BMP4 knockout and control mice after ovariectomized. n = 4. (C) Change of body weight of ovariectomized female mice during the period of estradiol administration. n = 4. (D) Glucose concentrations during an intraperitoneal glucose tolerance test (n = 8) and quantification of AUG (area under curve) from ovariectomized BMP4 knockout and control mice. n = 6. (E) Glucose concentrations during an intraperitoneal insulin tolerance test (n = 7) and quantification of AUG from ovariectomized BMP4 knockout and control mice. n = 6. (F) Glucose concentrations during an intraperitoneal glucose tolerance test (n = 8) and quantification of AUG (area under curve) from ovariectomized BMP4 knockout and control mice treated with estradiol. n = 6. (G) Glucose concentrations during an intraperitoneal insulin tolerance test (n = 7) and quantification of AUG from ovariectomized BMP4 knockout and control mice treated with estradiol. n = 6.
3.7. The Correlation Between BMP4 Levels and Body Mass Index (BMI) Depended Upon Estrogen Level
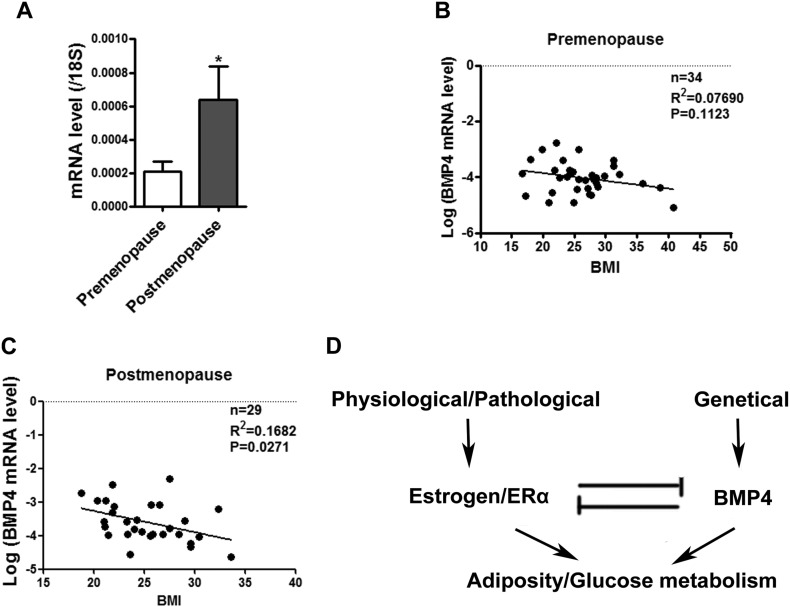
Our previous study showed that the level of BMP4 in human subcutaneous WAT from 32 participants (15 males, 17 females) correlated inversely with BMI(Qian et al., 2013), in the present study, more female participants were enrolled, and the females participants were grouped into premenopause and postmenopause (> 55 years old) groups. We found thatBMP4 levels in subcutaneous WAT significantly increased after menopause (Fig. 7A), and BMP4 levels inversely correlated with BMI in postmenopausal women (Fig. 7C). There was no significant correlation between BMP4 levels and BMI in premenopausal women, however, the data from individuals with BMI > 30 lead the correlation to show the similar trend (Fig. 7B).
Fig. 7.
The correlation between BMP4 level and body mass index (BMI) dependents on activation of estrogen/ERα. (A) Real-time PCR determined the BMP4 mRNA level in subcutaneous WAT of pre and post-menopausal women. (B&C) Linear regression analysis between BMI and BMP4 mRNA level in subcutaneous adipose tissue of premenopausal and postmenopausal women. (D) Schematic Model: Counterbalance between BMP4 and estrogen/ERα signaling in adipose tissue development and energy metabolism.
4. Discussion
Women have more subcutaneous fat and less visceral fat than men, and obesity and insulin resistance are less prevalent in young and middle-aged women but more prevalent in women over 60 (Yang et al., 2010). While their lower estrogen levels have been suggested to be a cause of the increase in obesity and insulin resistance among postmenopausal women, the underlying mechanism has yet to be clarified. Direct action of estrogens upon adipose tissue as well as indirect action through other factors may be involved (Meyer et al., 2011). Results from our previous study showed that BMP4 stimulates brown-like changes in WAT and improves insulin sensitivity (Qian et al., 2013). On the contrary, disruption of BMP4 in adipose tissue leads to obesity and insulin resistance in male mice (Qian et al., 2013). In this study, however we found that obesity and impairments in glucose metabolism did not occur in female BMP4 knockout mice. When challenged with HFD, female BMP4 knockout mice were protected from obesity. The difference between male and female mice in the effect of BMP4 on adiposity and energy balance suggested the involvement of interactions between BMP4 and estrogen signaling.
Our results showed that BMP4 knockout enhanced ERα levels in WAT. BMP4 is a member of the transforming growth factor β (TGF β) superfamily. Two major signaling pathways, the SMAD (mothers against decapentaplegic Drosophila homologue) pathway and p38 MAP kinase pathway convey most biological functions of BMP4 (Bragdon et al., 2011). Although there is no report on the regulation of ERα by BMP4, interactions between ERα and TGFβ had been studied in breast cancer cells (Band and Laiho, 2011, Ito et al., 2010, Stope et al., 2010), in which TGFβ was shown to have anti-estrogen activity, possibly by increasing proteasome-dependent degradation of ERα (Petrel and Brueggemeier, 2003). Considering that TGFβ and BMP4 share some of signaling partners, BMP4 is also very likely to be implicated in the regulation of ERα protein in adipocytes. In the present study, we found that activation by BMP4 stimulated the degradation of ERα through the proteasomal pathway (Fig. 3F), however the mechanism by which BMP4 meditates proteasomal degradation of ERα in WAT remains to be explored.
In young female mice, with normal levels of circulating estrogens, ERα signaling was activated as indicated by increased transcription of the reported ERα target genes. One of the tested genes-GPX3 plays important roles in metabolism. It was reported that the enzyme activity of GPX3 is negatively correlated to BMI (Olusi, 2002). GPX3 mRNA level was shown to increase in women with weight loss (Dahlman et al., 2005). LXRα and LXRβ are also targets of ERα. However mRNA of LXRα and LXRβ was not significantly enhanced in subcutaneous WAT. Because LXR genes have a complex regulation, there might be more factors participating in their expression. The activation of ERα signaling resulting from the knockout of BMP4 prevented overt adiposity and insulin resistance. As predicted, failure of activation of ERα due to aging and ovariectomy in female knockout mice (Supplementary Fig. 4) yielded a phenotype similar to that of male mice. Notably, loss of estrogens up-regulated the BMP4 level in WAT, and estrogens suppressed BMP4 expression through ERα signaling (Fig. 4F and G). The inhibitory effect of estrogens on BMP4 expression was also reported in other cell types, including cardiomyocytes (Wang et al., 2014) and submucosal smooth muscle cells in the adult female reproductive tract (Bhattacherjee et al., 2013).
Our results showed that BMP4 inhibited ERα signaling by increasing the degradation of ERα, and that the expression of BMP4 itself was suppressed by estrogens. This highlights the complexity of the regulatory network for adipocyte development and glucose metabolism. The crosstalk between BMP4 and estrogen/ ERα suggests a homeostatic balance between these two factors (Fig. 7D), with loss of either one resulting in the activation of the other to compensate for the defect.
The level of estrogens is age-dependent in females, whereas the expression of BMP4 might be influenced by genetics and may be highly variable among humans. It was reported that one genetic variant of BMP4 (single nucleotide polymorphism rs8014363) was associated with levels of triglyceride and high-density lipoprotein cholesterol after adjusting for age, gender, and BMI in a study that involved 6822 participants (Tang et al., 2013), supporting a link between BMP4 genetics and adipose tissue metabolism. Our previous study also showed that the level of BMP4 in human adipose tissues of about 32 participants (15 males and 17 females) correlated inversely with BMI (Qian et al., 2013). With more female participants enrolled in this study, we found that the inverse correlation was significant among postmenopausal women rather than premenopausal ones. Estrogens are an important sex hormone in females that decreases with age and in some pathological conditions, and when the regulation of BMP4 becomes dominant, which affect the incidence of obesity (Fig. 7D). This study also suggests that the regulation of BMP4 may offer a new opportunity of intervention in the control of excessive obesity.
The following are the supplementary data related to this article.
List of primers used in the study.
Funding sources
This research is supported partially by National Key Basic Research Project Grant 2011CB910201 and 2013CB530601, The State Key Program of National Natural Science Foundation 31030048C120114 and 81390350 (for Q.Q.T.); National Natural Science Foundation (Grant No. 81200621; for S.W.Q.). The Department is supported by Shanghai Leading Academic Discipline Project B110 and 985 Project 985-YFX0302.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author contributions
S.W.Q., Y.L., and Q.Q.T. designed research; S.W.Q., Y.L., J.W., J.C.N., M.Y.W., Y.T. and Y.X.Z. performed research; X.S.L. recruited human subjects S.W.Q., Y.L., and Q.Q.T. analyzed data; X.L., H.Y.H., G.L., X.S.L., and X.C.J. aided with data analysis; S.W.Q., Y.L., and Q.Q.T. wrote the paper. Q.Q.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
We thank Dr. Brigid Hogan at Department of Cell Biology, Duke University Medical Center for kindly providing BMP4LoxP/LoxP mice.
References
- Band A.M., Laiho M. Crosstalk of TGF-beta and estrogen receptor signaling in breast cancer. J. Mammary Gland Biol. Neoplasia. 2011;16:109–115. doi: 10.1007/s10911-011-9203-7. [DOI] [PubMed] [Google Scholar]
- Barros R.P., Gustafsson J.A.K. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Bhattacherjee A., Rumi M.A., Staecker H., Smith P.G. Bone morphogenetic protein 4 mediates estrogen-regulated sensory axon plasticity in the adult female reproductive tract. J. Neurosci. 2013;33:1050–1061. doi: 10.1523/JNEUROSCI.1704-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E., De Robertis E.M. Embryo development. BMP gradients: a paradigm for morphogen-mediated developmental patterning. Science. 2015;348:a5838. doi: 10.1126/science.aaa5838. [DOI] [PubMed] [Google Scholar]
- Bragdon B., Moseychuk O., Saldanha S., King D., Julian J., Nohe A. Bone morphogenetic proteins: a critical review. Cell. Signal. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Chen J., Brown T.R., Russo J. Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 2009;1793:1128–1143. doi: 10.1016/j.bbamcr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman I., Linder K., Arvidsson N.E., Andersson I., Liden J., Verdich C., Sorensen T.I., Arner P. Changes in adipose tissue gene expression with energy-restricted diets in obese women. Am. J. Clin. Nutr. 2005;81:1275–1285. doi: 10.1093/ajcn/81.6.1275. [DOI] [PubMed] [Google Scholar]
- Deng H.W., Li J., Li J.L., Dowd R., Davies K.M., Johnson M., Gong G., Deng H., Recker R.R. Association of estrogen receptor-alpha genotypes with body mass index in normal healthy postmenopausal Caucasian women. J. Clin. Endocrinol. Metab. 2000;85:2748–2751. doi: 10.1210/jcem.85.8.6728. [DOI] [PubMed] [Google Scholar]
- Elbers J.M., de Jong S., Teerlink T., Asscheman H., Seidell J.C., Gooren L.J. Changes in fat cell size and in vitro lipolytic activity of abdominal and gluteal adipocytes after a one-year cross-sex hormone administration in transsexuals. Metabolism. 1999;48:1371–1377. doi: 10.1016/s0026-0495(99)90146-4. [DOI] [PubMed] [Google Scholar]
- Enzi G., Gasparo M., Biondetti P.R., Fiore D., Semisa M., Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am. J. Clin. Nutr. 1986;44:739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- Fox C.S., Yang Q., Cupples L.A., Guo C.Y., Atwood L.D., Murabito J.M., Levy D., Mendelsohn M.E., Housman D.E., Shearman A.M. Sex-specific association between estrogen receptor-alpha gene variation and measures of adiposity: the Framingham Heart Study. J. Clin. Endocrinol. Metab. 2005;90:6257–6262. doi: 10.1210/jc.2005-0670. [DOI] [PubMed] [Google Scholar]
- Gambineri A., Pelusi C., Vicennati V., Pagotto U., Pasquali R. Obesity and the polycystic ovary syndrome. Int. J. Obes. Relat. Metab. Disord. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- Geer E.B., Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009;6(Suppl. 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine P.A., Taylor J.A., Iwamoto G.A., Lubahn D.B., Cooke P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B.L. Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Huang H., Song T.J., Li X., Hu L., He Q., Liu M., Lane M.D., Tang Q.Q. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. U. S. A. 2009;106:12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I., Hanyu A., Wayama M., Goto N., Katsuno Y., Kawasaki S., Nakajima Y., Kajiro M., Komatsu Y., Fujimura A. Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J. Biol. Chem. 2010;285:14747–14755. doi: 10.1074/jbc.M109.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.E., Thorburn A.W., Britt K.L., Hewitt K.N., Wreford N.G., Proietto J., Oz O.K., Leury B.J., Robertson K.M., Yao S. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.R., Clegg D.J., Prossnitz E.R., Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxford) 2011;203:259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misso M.L., Murata Y., Boon W.C., Jones M.E., Britt K.L., Simpson E.R. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144:1474–1480. doi: 10.1210/en.2002-221123. [DOI] [PubMed] [Google Scholar]
- Ohlsson C., Hellberg N., Parini P., Vidal O., Bohlooly-Y M., Rudling M., Lindberg M.K., Warner M., Angelin B., Gustafsson J.A. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem. Biophys. Res. Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- Okura T., Koda M., Ando F., Niino N., Ohta S., Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int. J. Obes. Relat. Metab. Disord. 2003;27:1020–1027. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- Olusi S.O. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int. J. Obes. Relat. Metab. Disord. 2002;26:1159–1164. doi: 10.1038/sj.ijo.0802066. [DOI] [PubMed] [Google Scholar]
- Petrel T.A., Brueggemeier R.W. Increased proteasome-dependent degradation of estrogen receptor-alpha by TGF-beta1 in breast cancer cell lines. J. Cell. Biochem. 2003;88:181–190. doi: 10.1002/jcb.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S.W., Tang Y., Li X., Liu Y., Zhang Y.Y., Huang H.Y., Xue R.D., Yu H.Y., Guo L., Gao H.D. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P.E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- Shi H., Clegg D.J. Sex differences in the regulation of body weight. Physiol. Behav. 2009;97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth S., Heron A. Diabetes and obesity: the twin epidemics. Nat. Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- Stope M.B., Popp S.L., Knabbe C., Buck M.B. Estrogen receptor alpha attenuates transforming growth factor-beta signaling in breast cancer cells independent from agonistic and antagonistic ligands. Breast Cancer Res. Treat. 2010;120:357–367. doi: 10.1007/s10549-009-0393-2. [DOI] [PubMed] [Google Scholar]
- Takeda K., Toda K., Saibara T., Nakagawa M., Saika K., Onishi T., Sugiura T., Shizuta Y. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J. Endocrinol. 2003;176:237–246. doi: 10.1677/joe.0.1760237. [DOI] [PubMed] [Google Scholar]
- Tang S., Zhang R., Yu W., Jiang F., Wang J., Chen M., Peng D., Yan J., Bao Y., Jia W. Association of genetic variants of BMP4 with type 2 diabetes mellitus and clinical traits in a Chinese Han population. Biomed. Res. Int. 2013;2013:238150. doi: 10.1155/2013/238150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M.J., Poehlman E.T., Matthews D.E., Tchernof A., MacCoss M.J. Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am. J. Physiol. Endocrinol. Metab. 2001;280:E496–E501. doi: 10.1152/ajpendo.2001.280.3.E496. [DOI] [PubMed] [Google Scholar]
- Vistisen D., Witte D.R., Tabak A.G., Brunner E.J., Kivimaki M., Faerch K. Sex differences in glucose and insulin trajectories prior to diabetes diagnosis: the Whitehall II study. Acta Diabetol. 2014;51:315–319. doi: 10.1007/s00592-012-0429-7. [DOI] [PubMed] [Google Scholar]
- Wang Y.C., Xiao X.L., Li N., Yang D., Xing Y., Huo R., Liu M.Y., Zhang Y.Q., Dong L. Oestrogen inhibits BMP4-induced BMP4 expression in cardiomyocytes: a potential mechanism of oestrogen-mediated protection against cardiac hypertrophy. Br. J. Pharmacol. 2014 doi: 10.1111/bph.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Lu J., Weng J., Jia W., Ji L., Xiao J., Shan Z., Liu J., Tian H., Ji Q. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of primers used in the study.