Abstract
Objective
To determine whether quiet standing measures at specific frequency levels (representative of reactive control) differed between individuals with stroke based on their ability to recover balance (failed or successful responses to external perturbations).
Methods
Individuals with stroke completed a clinical assessment, including 30 s of quiet standing and lean-and-release postural perturbations, at admission to in-patient rehabilitation. Quiet standing centre of pressure (COP) signals were calculated and discrete wavelet decomposition was performed. Net COP amplitude, between-limb synchronization, and ratios of individual-limb COP were determined for each frequency level of interest, and for the non-decomposed signal (all frequency levels). Outcome measures were compared between individuals who exhibited failed and successful responses during a) unconstrained and b) encouraged-use lean-and-release trials.
Results
Individuals with failed responses during the unconstrained lean-and-release trials displayed greater net COP amplitude than those with successful responses, specifically within a frequency range of 0.40–3.20 Hz.
Conclusions
Reduced ability to recover balance among individuals with stroke may be reflected in impaired reactive control of quiet standing.
Significance
These results provide insight into the mechanism by which reactive control of quiet standing is impaired in individuals with stroke, and may inform assessment and rehabilitation strategies for post-stroke reactive balance control.
Keywords: Stroke, quiet standing, ability to recover balance, discrete wavelet analysis, centre of pressure
1.0 Introduction
Falls risk is increased in individuals with stroke compared to age-matched healthy individuals, and falls are one of the most frequent complications experienced during post-stroke recovery (Batchelor et al., 2012). Up to 73% of individuals with stroke fall following their return to community living after discharge from rehabilitation (Weerdesteyn et al., 2008), and individuals with stroke are 2–4 times more likely to suffer a fall-related injury compared to age-matched controls (O’Loughlin et al., 1993; Graafmans et al., 1996; Jørgensen et al., 2002). Falls occur when an individual fails to recover from a loss of balance (Maki and McIlroy, 1996), which could potentially result from either external or internal postural perturbations.
A step is one of several possible responses to postural perturbations. Stepping allows the individual to regain stability by re-capturing the moving centre of mass (COM) within the base of support (Maki and McIlroy, 1997; Maki et al., 2003; Maki and McIlroy, 2005). The ability to execute compensatory steps following a perturbation is critical in maintaining balance and mobility (Maki and McIlroy, 1997) and in preventing falls (Holliday et al., 1990; Hyndman et al., 2002). Stepping reactions are often impaired in individuals with stroke, such that steps in response to a perturbation tend to be characterized by an increased need for external assistance, inability to step with either limb, and/or increased occurrence of multiple-step responses (Lakhani et al., 2011; Inness et al., 2014). Furthermore, impaired stepping in individuals with stroke has been related to the occurrence of falls during (Mansfield et al., 2013) and following (Mansfield et al., 2015) discharge from in-patient rehabilitation.
The control of quiet standing is also impaired post-stroke, in that individuals with stroke exhibit greater net centre of pressure (COP) amplitude relative to healthy controls (Mansfield et al., 2011). In addition, there is a trend towards reduced temporal synchronization between the COP of the left and right limbs among individuals with stroke compared to healthy controls (Mansfield et al., 2011). Increased COP amplitude in quiet standing is associated with increased falls risk in older adults (Maki et al., 1994). Similarly, reduced between-limb synchronization is related to increased falls risk during (Mansfield et al., 2012) and following discharge from inpatient rehabilitation (Mansfield et al., 2015) among individuals with stroke.
Previous studies have examined all frequencies present in the COP time series collectively (Mansfield et al., 2011; Mansfield et al., 2012; Mansfield et al., 2015), providing a global indication of quiet standing control (Singer and Mochizuki, 2015). However, previous models have suggested two main frequency components within the COP signal, above and below approximately 0.4 Hz (Winter et al., 1998; Zatsiorsky and Duarte, 1999; Zatsiorsky and Duarte, 2000). The lower frequency components may represent an anticipatory control mechanism, indicative of COM dynamics, exploratory COP migrations, or errors in state estimation (Winter et al., 1998; Latash et al., 2003; Kiemel et al., 2006; Carpenter et al., 2010); while the higher frequency components may be representative of reactive control, specifically balance corrections executed in response to transient instability (Paillex and So, 2003; Singer and Mochizuki, 2015). Frequency decomposition of the COP signal may provide more detailed information regarding reactive versus anticipatory control of quiet standing in individuals with stroke (Singer and Mochizuki, 2015), and insight into mechanisms by which reactive control of quiet standing is impaired, and fall risk subsequently increased, in this population.
To our knowledge, no study to date has examined the reactive control of quiet standing as a function of ability to recover balance in individuals with stroke. This study aimed to determine whether quiet standing measures at specific frequency levels within the COP signal differed based on ability to recover balance in individuals with stroke. The same measures, calculated for the non-decomposed COP signal (all frequency levels), were also compared between groups. It was hypothesized that compared to those with successful balance recovery reactions, individuals with failed reactions would exhibit greater COP amplitude, reduced between-limb synchronization, and ratios between the individual-limb COP amplitudes that were further from 0.50; these differences would be identified at frequencies representative of reactive control (>0.4 Hz), but not those representative of anticipatory control (<0.4 Hz).
2.0 Methods
2.1 Participants
This study consisted of a retrospective analysis of data from individuals with stroke who were enrolled in an in-patient stroke rehabilitation program between October 2009 and September 2012. For inclusion in the analysis, a clinical assessment must have been conducted at admission by a physiotherapist (part of routine care), during which participants must have completed a 30 s trial of eyes-open quiet standing, as well as five ‘unconstrained’ trials with a lean-and-release system to assess ability to recover balance (see Section 2.2). These criteria were met by 84 of 512 individuals (16%). All procedures were approved by the Toronto Rehabilitation Institute Research Ethics Board (approval number: TRI-REB #10-046) with a waiver of patient consent approved for the purpose of the review.
2.2 Assessments
2.2.1 Demographic Information
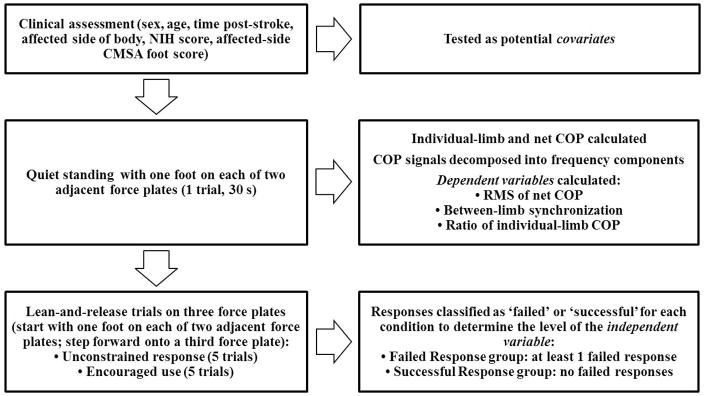
Participants’ hospital charts were used to determine sex, age, time post-stroke, affected side of the body, National Institutes of Health Stroke Scale (NIHSS) score, and Chedoke-McMaster Stroke Assessment (CMSA) foot score for the affected side of the body (Figure 1). These variables were assessed as potential covariates in the analysis (see Section 2.4). Stroke type and presence/absence of peripheral neuropathy, vestibular conditions, visual field deficit, and neglect were also collected from hospital charts to characterize the study sample (Table 1).
Figure 1.
Data collection procedures and information gained from each component of the assessment.
Table 1.
Demographic and stroke-related characteristics of the Failed Response and Successful Response groups, based on a) unconstrained and b) encouraged-use lean-and-release trials. *Characteristics tested as covariates.
| Characteristic | Failed Response group | Successful Response group | p-value | |
|---|---|---|---|---|
| Groups based on unconstrained lean-and-release trials | N=29 | N=55 | ||
|
| ||||
| Sex (number (%))* | Men | 19 (65.5%) | 46 (83.6%) | 0.059 |
| Women | 10 (34.5%) | 9 (16.4%) | ||
| Age (years)* | 66.8 (14.7) | 64.3 (12.9) | 0.43 | |
| NIHSS scorea* | 3.8 (2.6) | 2.8 (2.7) | 0.17 | |
| CMSA foot score (more-affected side)b* | 4.2 (1.2) | 4.7 (1.1) | 0.073 | |
| Time since stroke (days)* | 23.1 (16.2) | 21.1 (11.0) | 0.50 | |
| More-affected side of the body (number (%))* | Right | 9 (31.0%) | 36 (65.5%) | 0.75 |
| Left | 20 (69.0%) | 19 (34.5%) | ||
| Stroke type (number (%)) | Ischemic | 23 (79.3%) | 46 (83.6%) | N/A |
| Haemorrhagic | 6 (20.7%) | 6 (10.9%) | ||
| Transforming to haemorrhagic | 0 (0.0%) | 2 (3.6%) | ||
| N/A | 0 (0.0%) | 1 (1.8%) | ||
| History of or current peripheral neuropathy (number (%)) | Present | 1 (3.4%) | 3 (5.5%) | N/A |
| Absent | 28 (96.6%) | 52 (94.5%) | ||
| History of or current vestibular condition (number (%)) | Present | 1 (3.4%) | 3 (5.5%) | N/A |
| Absent | 28 (96.6%) | 52 (94.5%) | ||
| History of or current visual field deficit (number (%)) | Present | 3 (10.3%) | 6 (10.9%) | N/A |
| Absent | 26 (89.7%) | 49 (89.1%) | ||
| History of or current neglect (number (%)) | Present | 6 (20.7%) | 7 (12.7%) | N/A |
| Absent | 23 (79.3%) | 48 (87.3%) | ||
|
| ||||
| Groups based on encouraged-use lean-and-release trials | N=43 | N=20 | ||
|
| ||||
| Sex (number (%))* | Men | 34 (79.1%) | 16 (80.0%) | 0.93 |
| Women | 9 (20.9%) | 4 (20.0%) | ||
| Age (years)* | 66.4 (13.1) | 59.5 (13.5) | 0.057 | |
| NIHSS scorec* | 3.6 (2.6) | 2.6 (2.8) | 0.21 | |
| CMSA foot score (more-affected side)* | 4.2 (1.0) | 5.3 (1.1) | <0.001 | |
| Time since stroke (days)* | 22.3 (14.1) | 21.6 (13.5) | 0.86 | |
| More-affected side of the body (number (%))* | Right | 28 (65.1%) | 16 (80.0%) | 0.23 |
| Left | 15 (34.9%) | 4 (20.0%) | ||
| Stroke type (number (%)) | Ischemic | 33 (76.7%) | 19 (95.0%) | N/A |
| Haemorrhagic | 8 (18.6%) | 1 (5.0%) | ||
| Transforming to haemorrhagic | 1 (2.3%) | 0 (0.0%) | ||
| N/A | 1 (2.3%) | 0 (0.0%) | ||
| History of or current peripheral neuropathy (number (%)) | Present | 2 (4.7%) | 1 (5.0%) | N/A |
| Absent | 41 (95.3%) | 19 (95.0%) | ||
| History of or current vestibular condition (number (%)) | Present | 2 (4.7%) | 2 (10.0%) | N/A |
| Absent | 41 (95.3%) | 18 (90.0%) | ||
| History of or current visual field deficit (number (%)) | Present | 2 (4.7%) | 4 (20.0%) | N/A |
| Absent | 41 (95.3%) | 16 (80.0%) | ||
| History of or current neglect (number (%)) | Present | 10 (23.3%) | 1 (5.0%) | N/A |
| Absent | 33 (76.7%) | 19 (95.0%) | ||
Data available for 68 participants (Failed Response group: N=22; Successful Response group: N=46);
Data available for 82 participants (Failed Response group: N=28; Successful Response group: N=54);
Data available for 52 participants (Failed Response group: N=36; Successful Response group: N=16).
2.2.2 Quiet Standing
As part of the clinical assessment, each participant performed one 30 s quiet standing trial with the eyes open. Ground reaction forces were recorded from two adjacent force plates (Advanced Mechanical Technology, Inc., Watertown, USA). Participants stood with one foot positioned on each plate, with a template used to ensure standardized foot position (McIlroy and Maki, 1997). No further instructions regarding weight bearing were provided. Force plate data were sampled at 256 Hz. COP signals for the quiet standing trials were calculated offline. Metrics determined from these signals were used as dependent variables in the analysis (see Sections 2.3 and 2.4).
2.2.3 Reactive Stepping
Participants also completed a lean-and-release test to assess ability to recovery balance (Mansfield et al., 2013). Participants wore a safety harness connected to an overhead frame to protect against a fall. A harness around the participant’s trunk was attached by a cable to a support beam behind the participant. At the beginning of each trial, the same template as described in Section 2.2.2 (McIlroy and Maki, 1997) was used to ensure that the feet were in a standardized position with one foot on each of two force plates. The participant then leaned against the release cable such that it supported approximately 10% of body weight. The cable was released at an unpredictable time, requiring the participant to take at least one step to prevent a fall. A third force plate was positioned in front of the other two force plates, such that participants generally stepped forward onto the third force plate. Ground reaction force data were collected throughout the lean-and-release trials as standard practice for the clinical balance assessment protocol. However, these data were not used for the purpose of the current analysis.
Five trials were performed in which participants were not constrained with respect to their stepping following the perturbation (‘unconstrained trials’). The limb used to initiate stepping on the majority of the five trials was designated as the preferred limb. Five additional trials were then performed in which the use of the non-preferred limb was encouraged for step initiation (‘encouraged-use trials’), by the physiotherapist physically blocking the preferred limb with their hand and/or foot, and by verbally encouraging the use of the non-preferred limb. Unconstrained trials assessed participants’ ability to respond to a postural perturbation when no restrictions were placed on responses (i.e. overall or general ability to recover balance), whereas encouraged-use trials assessed participants’ ability to step with either limb to regain stability (i.e. the stepping abilities of the individual limbs, specifically to reveal impairments in stepping with the non-preferred limb). All trials were videotaped and analyzed post-collection.
For participants to be included in the analysis based on the unconstrained trials, the amount of weight on the release cable during all five trials must have been at least 5% of body weight, to ensure a sufficient magnitude of perturbation. Based on the video data, participants were divided into two groups: Failed Response (N=29), in which participants required assistance during at least one of the five trials (where ‘assistance’ was defined as the participant requiring external support from the safety harness or physiotherapist, as determined by visual observation), and Successful Response (N=55), in which participants did not require assistance during any of the five trials. These groups constituted the independent variable in the analysis based on the unconstrained lean-and-release trials (see Section 2.4).
Of the 84 individuals who met the criteria for the unconstrained trials, those who also completed five encouraged-use trials with at least 5% of body weight on the release cable (N=63) were included in further analysis based on these trials. From the video data, participants were divided into two groups: Failed Response (N=43), in which the participant required assistance, stepped with the blocked/non-encouraged limb, or exhibited a slide step with the encouraged limb, as determined by visual observation; and Successful Response (N=20), in which the participant did not require assistance and exhibited a successful full step with the encouraged limb. These groups constituted the independent variable in the analysis based on the encouraged-use lean-and-release trials (see Section 2.4). The additional characteristics for a failed response were included for the encouraged-use trials as the aim of this condition was to reveal difficulties in stepping with the non-preferred limb specifically, as opposed to general ability to recover balance.
2.3 Data Processing
Force plate data from the quiet standing trials were low-pass filtered with a dual-pass, fourth order Butterworth filter with a cutoff frequency of 10 Hz (Hendrickson et al., 2014). COP signals were calculated for each limb separately and both limbs together (i.e. net COP), in the antero-posterior (AP) and medio-lateral (ML) directions, although only the AP data were analyzed as individual-limb AP COP is more important than individual-limb ML COP for overall balance control (Winter et al., 1993). Discrete wavelet decomposition (Rioul and Vetterli, 1991; Addison, 2005; Fryzlewicz, 2010) was conducted to examine the components of the signal contained within various frequency ranges, using a custom program written in Matlab v.8.4 (The MathWorks, Inc., Natick, USA). Wavelet decomposition was selected instead of a short-time Fourier transform as the resolution of the latter is fixed, while the former enables improved frequency resolution at lower frequencies and improved time resolution at higher frequencies (Singer and Mochizuki, 2015). An eleven-level decomposition (D1–D11, A11; Table 2) was performed on the left, right, and net COP signals (Singer and Mochizuki, 2015). For the frequency ranges of interest (spanning a range from 0.050 Hz to 3.20 Hz), the root mean square (RMS) of the net COP signal was calculated at each frequency range of interest, to represent COP amplitude. Between-limb synchronization was quantified by cross-correlating the left and right limb COP signals, and extracting the cross-correlation coefficient at zero time lag. The ratio of the RMS of the COP under the individual limbs was calculated as [(RMS of preferred limb)/(RMS of left limb + RMS of right limb)]. All measures were also calculated for the non-decomposed COP signal (all frequency levels).
Table 2.
Decomposition levels and corresponding frequency ranges. Shaded cells indicate frequency ranges of interest (frequencies >3.20Hz are assumed to represent noise). The frequency ranges for levels D6–D8 (higher frequencies; bolded) are thought to be representative of reactive control, while the frequency ranges for levels D9–D11 (lower frequencies; italicized) are thought to be representative of anticipatory control.
| Decomposition level | Frequency range |
|---|---|
| D1 | 51.25–102.50Hz |
| D2 | 25.63–51.25Hz |
| D3 | 12.81–25.63Hz |
| D4 | 6.41–12.81Hz |
| D5 | 3.20–6.41Hz |
| D6 | 1.60–3.20Hz |
| D7 | 0.80–1.60Hz |
| D8 | 0.40–0.80Hz |
| D9 | 0.20–0.40Hz |
| D10 | 0.10–0.20Hz |
| D11 | 0.050–0.10Hz |
| A11 | DC–0.050Hz |
| Total | Non-decomposed signal |
2.4 Data Analysis
All outcome measures (RMS of the net COP signal, between-limb synchronization, and ratio of the individual-limb RMS, for both the non-decomposed signal and at each frequency level) were tested for normality using the Shapiro-Wilk test. For outcome measures in which the distribution was non-normal (all net COP amplitude and between-limb synchronization measures), a rank transformation was applied prior to analysis. For each outcome measure for both the non-decomposed signal and at each frequency level, demographic and stroke-related measures were tested as potential covariates, including sex, age, NIHSS score, CMSA foot score, time since stroke, and affected side of the body. For continuous measures, the criteria to be included as a covariate were: a moderate correlation to the outcome measure (Pearson product moment correlation, |r|>0.40), and a difference between the Failed Response and Successful Response groups (independent T-test, p<0.15). Nominal measures were included as a covariate if there was a difference in the outcome measure between levels of the covariate (independent T-test, p<0.15), and if the potential covariate differed between the Failed Response and Successful Response groups (Chi square, p<0.15). As participants were tested as part of routine clinical care, and were not recruited according to specific criteria, it was important to account for extraneous factors to the greatest possible extent. Therefore, an alpha level of 0.15 was chosen as a liberal approach to covariate identification and to increase the likelihood of identifying all relevant covariates.
Two sets of one-way analyses of (co-)variance comparing the outcome measures between the Failed Response and Successful Response groups were conducted; in one set, the groups were based on the responses from the unconstrained lean-and-release trials, and in the other set, the encouraged-use trials. Analyses were based on both types of trials due to the different information that each provided with respect to ability to recover balance. The former provided an indication of differences in the quiet standing outcome measures based on overall ability to recover balance; while the latter provided more specific information about the stepping ability of the non-preferred limb, to determine whether the outcome measures were being influenced by one of the limbs to a greater extent. Correspondingly, the RMS of the net COP signal (global measure) was compared between the Failed Response and Successful Response groups determined based on the unconstrained lean-and-release trials, while the between-limb synchronization and individual-limb RMS ratios (limb-specific measures) were compared between the Failed Response and Successful Response groups determined based on the encouraged-use lean-and-release trials. Alpha was adjusted for multiple comparisons from p<0.05 using the Holm-Bonferroni method (Holm, 1979). Effect sizes for each outcome measure were reported as Hedges’ g (a measure of standardized mean difference in which Cohen’s d is corrected for bias due to sample averages (Lakens, 2013)), and as partial eta squared (ηp2) for comparison to previous work (Singer and Mochizuki, 2015). For outcome measures in which an analysis of co-variance was conducted, violation of the assumption of homogeneity of regression slopes was addressed by including the covariate as a factor in the model. This resulted in a 2-by-2 analysis of variance (Failed Response or Successful Response group; factor that was originally a covariate).
3.0 Results
3.1 Non-decomposed signal measures
Affected-side CMSA foot score was a covariate for between-limb synchronization for the non-decomposed signal when the groups were determined based on performance during the encouraged-use lean-and-release trials (Table 1). The assumption of homogeneity of regression slopes was violated for the analysis of this outcome measure. Therefore, participants were grouped according to their affected-side CMSA foot score (4 or less, requiring physical contact from the assessing physiotherapist; 5 or greater, requiring no physical contact), and this factor was included in the analysis of variance model, resulting in a 2-by-2 ANOVA for this outcome measure. There were no differences in any of the non-decomposed signal measures between the Failed Response and Successful Response groups based on either the unconstrained or encouraged-use trials (Table 3).
Table 3.
Test statistics and effect size for each outcome measure.
| Outcome Measure | One-way analysis of (co-)variance | Two-way analysis of variance | Effect size (Hedges’ g [95% confidence interval]; partial eta squared (ηp2)) |
|---|---|---|---|
| Amplitude of net centre of pressure (root mean square; cm)○ | |||
|
| |||
| D6 (1.60–3.20Hz) | F1,82=10.37, p=0.002* | 0.88 [0.17, 1.59]; 0.11 | |
| D7 (0.80–1.60Hz) | F1,82=13.71, p<0.002* | 0.88 [0.17, 1.60]; 0.14 | |
| D8 (0.40–0.80Hz) | F1,82=9.88, p=0.002* | 0.78 [0.083, 1.47]; 0.11 | |
| D9 (0.20–0.40Hz) | F1,80=0.58, p=0.45 | 0.72 [0.028, 1.41]; 0.007 | |
| Group–covariate interaction (sex) | F2,80=2.28, p=0.11 | ||
| D10 (0.10–0.20Hz) | F1,82=1.74, p=0.19 | 0.16 [−0.39, 0.71]; 0.021 | |
| D11 (0.050–0.10Hz) | F1,82=1.79, p=0.19 | 0.15 [−0.39, 0.70]; 0.021 | |
| Non-decomposed signal | F1,82=5,68, p=0.019 | 0.45 [−0.18, 1.07]; 0.065 | |
|
| |||
| Between-limb synchronization (cross-correlation coefficient at zero time lag)■ | |||
|
| |||
| D6 (1.60–3.20Hz) | F1,59=2.17, p=0.15 | Group: F1,59=3.31, p=0.074 | 0.50 [−0.14, 1.15]; 0.053 |
| Group–covariate interaction (CMSA foot score) | F2,59=7.23, p=0.002Δ | CMSA: F1,59=7.30, p=0.009 | |
| Interaction: F1,59=0.63, p=0.43 | |||
| D7 (0.80–1.60Hz) | F1,59=1.63, p=0.21 | Group: F1,59=1.64, p=0.21 | 0.54 [−0.13, 1.20]; 0.027 |
| Group–covariate interaction (CMSA foot score) | F2,59=5.90, p=0.005Δ | CMSA: F1,59=4.96, p=0.030 | |
| Interaction: F1,59=0.001, p=0.97 | |||
| D8 (0.40–0.80Hz) | F1,59=0.14, p=0.71 | Group: F1,59=0.30, p=0.58 | 0.060 [−0.46, 0.58]; 0.005 |
| Group–covariate interaction (CMSA foot score) | F2,59=7.22, p=0.002Δ | CMSA: F1,59=7.63, p=0.008 | |
| Interaction: F1,59=0.10, p=0.75 | |||
| D9 (0.20–0.40Hz) | F1,61=0.76, p=0.39 | 0.24 [−0.33, 0.71]; 0.012 | |
| D10 (0.10–0.20Hz) | F1,61=11.45, p=0.001* | 0.46 [−0.16, 1.08]; 0.16 | |
| D11 (0.050–0.10Hz) | F1,61=0.23, p=0.63 | 0.089 [−0.43, 0.61]; 0.004 | |
| Non-decomposed signal | F1,59=5.00, p=0.029 | Group: F1,59=4.16, p=0.046 | 0.11 [−0.42, 0.64]; 0.066 |
| Group–covariate interaction (CMSA foot score) | F1,59=6.52, p=0.003Δ | CMSA: F1,59=0.82, p=0.37 | |
| Interaction: F1,59=4.63, p=0.036 | |||
|
| |||
| Ratio of individual-limb root mean square■ | |||
|
| |||
| D6 (1.60–3.20Hz) | F1,61=1.80, p=0.18 | 0.36 [−0.25, 0.96]; 0.029 | |
| D7 (0.80–1.60Hz) | F1,61=1.23, p=0.27 | 0.30 [−0.29, 0.88]; 0.020 | |
| D8 (0.40–0.80Hz) | F1,61=2.05, p=0.16 | 0.38 [−0.23, 0.99]; 0.032 | |
| D9 (0.20–0.40Hz) | F1,61=2.75, p=0.10 | 0.44 [−0.19, 1.07]; 0.043 | |
| D10 (0.10–0.20Hz) | F1,61=3.18, p=0.08 | 0.48 [−0.16, 1.12]; 0.050 | |
| D11 (0.050–0.10Hz) | F1,61=0.51, p=0.48 | 0.19 [−0.36, 0.75]; 0.008 | |
| Non-decomposed signal | F1,61=1.73, p=0.19 | 0.35 [−0.25, 0.96]; 0.028 | |
Groups determined based on unconstrained lean-and-release trials;
Groups determined based on encouraged-use lean-and-release trials;
Significant effect of the factor of Group (Holm-Bonferroni adjusted alpha for groups based on unconstrained lean-and-release trials, α=0.007; for groups based on encouraged-use lean-and-release trials, α=0.004);
Significant interaction between the factor of Group and the covariate.
3.2 Decomposed signal measures - unconstrained lean-and-release trials
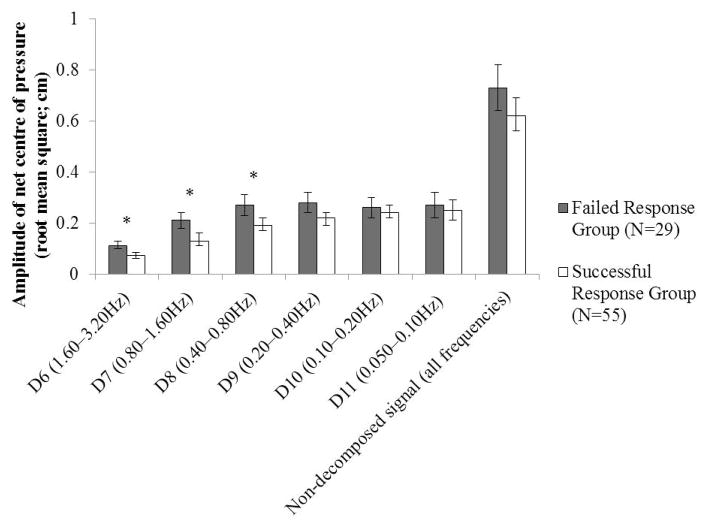
Sex was a covariate for RMS of the net COP at the D9 level. Following Holm-Bonferroni adjustment, the RMS of the net COP at the D6, D7, and D8 levels (0.40–3.20 Hz) was significantly higher in the Failed Response group compared to the Successful Response group (p≤0.002) (Figure 2). Effect sizes for these measures ranged from 0.78–0.88 (Hedges’ g) and from 0.11–0.14 (ηp2).
Figure 2.
Mean (95% confidence interval) RMS of net COP values for each level and for the non-decomposed signal, compared between the Failed Response and Successful Response groups (determined based on the unconstrained lean-and-release trials). *Significant following Holm-Bonferroni adjustment (α=0.007).
3.3 Decomposed signal measures - encouraged-use lean-and-release trials
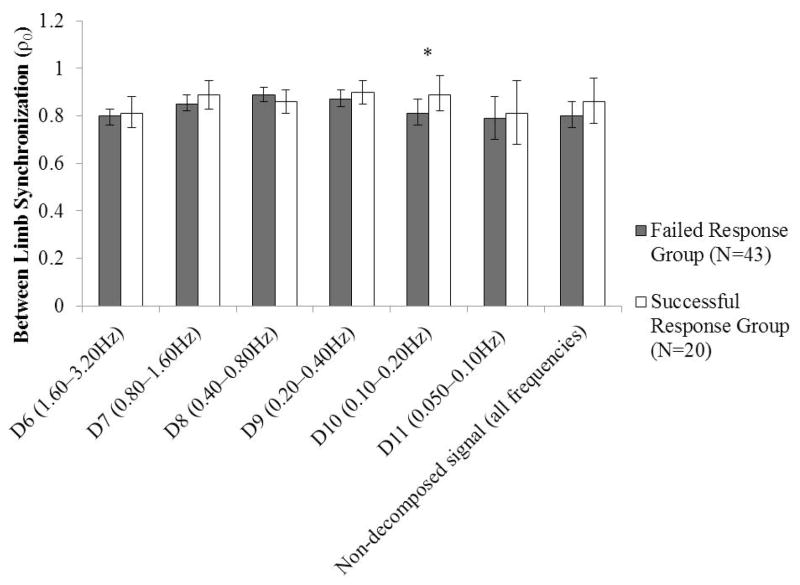
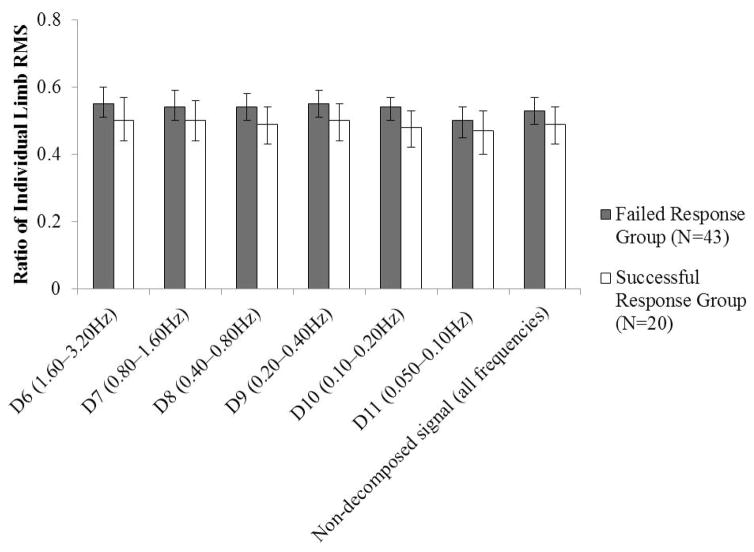
Affected-side CMSA foot score was a covariate for between-limb synchronization at the D6, D7, and D8 levels. The assumption of homogeneity of regression slopes was violated for each of these analyses, and therefore affected-side CMSA score was included as a factor in the analysis of variance model as described in Section 3.1. For all outcome measures compared on the basis of the encouraged-use lean-and-release trials, the only measure for which a significant difference was identified between groups was between-limb synchronization at the D10 level (Hedges’ g=0.46; ηp2=0.16), such that between-limb synchronization was lower in the Failed Response group than in the Successful Response group (Figures 3 and 4). For the remaining comparisons between outcome measures, the mean effect sizes were Hedges’ g=0.31 (range: 0.06–0.54) and ηp2=0.029 (range: 0.004–0.066). However, the direction of the differences was generally in the hypothesized direction (reduced between-limb synchronization, ratios between the individual-limb COP amplitudes that were further from 0.50 for the Failed Response group).
Figure 3.
Mean (95% confidence interval) between-limb synchronization for each level and for the non-decomposed signal, compared between the Failed Response and Successful Response groups (determined based on the encouraged-use lean-and-release trials). *Significant following Holm-Bonferroni adjustment (α=0.004).
Figure 4.
Mean (95% confidence interval) individual-limb RMS ratios for each level and for the non-decomposed signal, compared between the Failed Response and Successful Response groups (determined based on the encouraged-use lean-and-release trials). The Holm-Bonferroni corrected alpha was 0.004.
4.0 Discussion
In support of our hypothesis, compared to those who exhibited successful responses, increased net COP amplitude at frequencies >0.4 Hz were identified in individuals who exhibited failed responses following a large-magnitude postural perturbation in which responses were unconstrained (i.e. preferred stepping limb). These findings may be indicative of impaired reactive balance control of quiet standing in individuals with reduced ability to recover balance. Although it was hypothesized that no differences would be identified in frequency levels representative of anticipatory control, between-limb synchronization at the D10 level differed between groups when determined based on the encouraged-use trials (i.e. non-preferred stepping limb). No significant differences were observed in the remaining between-limb synchronization or ratio measures.
In the study of quiet standing and postural control, wavelet decomposition has been applied to COP signals to compare clinical groups and healthy controls (Morales and Kolaczyk, 2002; Chagdes et al., 2009; Suarez et al., 2013; Clark et al., 2014). Furthermore, wavelet decomposition has been used to investigate differences between individuals post-stroke with and without spasticity (Singer and Mochizuki, 2015). As wavelet decomposition appears to distinguish between clinical populations and healthy controls (Morales and Kolaczyk, 2002; Chagdes et al., 2009; Prosser et al., 2010; Suarez et al., 2013; Clark et al., 2014), as well as between sub-groups within a clinical population (Singer and Mochizuki, 2015), this technique may be a promising tool for clinical analysis. Metrics of the non-decomposed COP signal have previously been analyzed to identify differences post-stroke (Mansfield et al., 2011) and associations with falling (Mansfield et al., 2012; Mansfield et al., 2015). However, the use of a wavelet approach to investigate relationships between ability to recover balance and reactive control of quiet standing is limited. The need to decompose the COP signal is supported by the lack of significant differences in the non-decomposed signal (all frequency levels) between groups in the present study, as well as previous work that found no relationship between quiet standing control and ability to recover balance in response to perturbations (Maki et al., 1990; Owings et al., 2000; Mackey and Robinovitch, 2005). The lack of significant relationships between quiet standing control and ability to recover balance following an external postural perturbation in past work may, in part, be attributable to a focus on the non-decomposed COP signal (as opposed to decomposing the signal into constituent frequency levels).
Existing models suggest that the control of quiet standing may be exerted through two different mechanisms. The lower-frequency COP levels may relate to an anticipatory control mechanism. Within the context of the present study, however, the primary focus was the higher-frequency COP levels, which are thought to represent balance corrections in response to transient instability (Paillex and So, 2003; Singer and Mochizuki, 2015). Similarly, the lean-and-release tasks evaluated individuals’ ability to respond to more extreme instability in which necessary support was suddenly removed, requiring that they must quickly take at least one step to prevent a fall to the floor (although the safety harness or a physiotherapist ultimately prevented a fall). Therefore, the balance recovery abilities of the two groups of participants may also be reflected in the reactive control of quiet standing. This finding is supported by studies that aimed to identify the structures in the central nervous system that are involved in balance. Although the spinal cord and brain stem are likely involved in reactive balance (Jacobs and Horak, 2007), emerging evidence has shown that the cerebral cortex may also be involved in quiet standing (Parokaran Varghese et al., 2015) and recovery from external perturbations (Jacobs and Horak, 2007; Mochizuki et al., 2008; Mochizuki et al., 2010; Fujimoto et al., 2014). It is thought that the fronto-central region, specifically the supplementary motor area, may play a role in reactive balance (Jacobs and Horak, 2007). The basal ganglia and cerebellum may also be involved in the modulation of responses through the use of contextual information and past experience (Jacobs and Horak, 2007). Taken together with these past works, the present findings suggest the existence of a common pathway or control centre for reactive balance control, regardless of the magnitude of perturbation (i.e. small-magnitude internal perturbations such as those experienced during quiet standing, represented by the higher-frequency components of the COP signal, or large-magnitude external perturbations).
Significant differences, with effect sizes ranging from 0.78–0.88 (Hedges’ g) and from 0.11–0.14 (ηp2), were found within net COP amplitude measures when the Failed and Successful Response groups were determined based on performance in the unconstrained lean-and-release task. That factors such as stroke severity and motor impairment were tested and found to be unnecessary as covariates provides support that the findings were not simply the result of a common physical factor underlying performance on both assessments. The COP signal encompasses COM displacements in addition to the stabilizing moments produced around the ankle joints by the lower leg muscles (Winter et al., 1996; Geurts et al., 2005). The increased amplitude of the non-decomposed COP signal that is often observed post-stroke may result from increases in both COM displacement and stabilizing moments (Paillex and So, 2003; Geurts et al., 2005). Between the two groups of individuals with stroke in the present study, there were no differences in the COM dynamics (lower frequencies) when determined based on general stepping ability, and therefore no differences in postural stability. However, the greater COP amplitude at higher frequencies in the Failed Response group implies that these individuals were exerting more stabilizing moments (i.e. reactive correction) in order to constrain the COM dynamics. The greater degree of active correction required by the individuals in the Failed Response group to perform the simplest of the tasks in the present study (quiet standing) may suggest that a larger potential for instability exists when these individuals are perturbed, thereby contributing to the increased need for assistance required by these individuals.
The encouraged-use trials quantified the ability to recover balance with the non-preferred limb; and the limb-specific quiet standing measures (between-limb synchronization, ratio of individual-limb RMS) quantified the individual limbs’ function in relation to each other. Of the limb-specific measures, a difference between the Failed Response and Successful Response groups when based on the encouraged-use lean-and-release trials was only observed for between-limb synchronization at the D10 level (0.10–0.20 Hz; representative of anticipatory control). This may result from a deficit in one of the limbs, or in the ability to synchronize the COP movements under the individual limbs. Specifically, this finding may indicate that individuals in whom ability to recover balance is impaired when attempting to step with the non-preferred limb also experienced challenges in the temporal co-modulation of the lower-frequency COP movements. While significant differences in between-limb synchronization were not identified in the higher-frequency components, the non-significant trends aligned with those previously identified between individuals with stroke, with and without unilateral spasticity (Singer and Mochizuki, 2015). Furthermore, partial eta squared values for between-limb synchronization were within the range of those previously reported (Singer and Mochizuki, 2015). Potentially, between-limb synchronization at frequencies representative of reactive control may be more sensitive to detecting differences in individuals with spasticity, in which discrepancies in motor control between the limbs may be more profound. Alternatively, the lack of differences between groups in the frequency levels representative of reactive control, when comparisons were made based on the encouraged-use trials, may suggest that membership in either group may be related to factors other than differences in the control of quiet standing. For example, cognitive or emotional factors such as anxiety or confidence related to stepping with the non-preferred limb (Lakhani et al., 2011) may have been unrelated to quiet standing ability, but influential in determining which group an individual was placed in.
The relevance of these findings for clinical practice lies in the previously established link between impaired ability to recover balance and fall risk, as an increased need for external assistance during compensatory stepping tasks has been shown to relate to fall risk during inpatient rehabilitation (Mansfield et al., 2013). As the present findings have suggested that a relationship may exist between the reactive control of quiet standing and ability to recover from an external postural perturbation, this study has provided the first step to linking the former to fall risk. The reactive control of quiet standing may also provide a potential surrogate measure for the assessment of ability to recover balance in a clinical setting. In the present dataset, although 240 participants completed the quiet standing assessment, 156 and 176 were not able to complete five unconstrained and encouraged-use lean-and-release trials, respectively, and therefore were discounted from the analysis. Similarly, a previous study found that lean-and-release assessments were generally not administered to patients with lower levels of function (Inness et al., 2015). Therefore, the use of a quiet standing assessment as a surrogate measure to evaluate ability to recover balance in a clinical setting could be beneficial in that it may be more tolerable for individuals with low levels of function, and can therefore be administered to a greater proportion of in-patients. Further research is required to confirm these findings, as well as to identify strategies to aid in improving reactive control of quiet standing and subsequently to determine whether these strategies also improve balance recovery.
The present study was limited by several methodological considerations. As the study design was cross-sectional, it was not possible from the present analysis to determine if the ability to recover balance assessed by the quiet standing and lean-and-release tests are measuring similar components of balance control, or if it is simply the case that individuals who show impairment on one measure are also more likely to show impairment on the other. Furthermore, as data were collected during clinical assessments conducted as a part of routine care, full datasets were not available for all participants. As such, it is possible that the present results do not represent patients at a lower level of function. However, this effect may have been somewhat tempered by the nature of the present analysis, as it was a retrospective review of data obtained as part of routine care. This may have enabled the inclusion of data from individuals who would otherwise not have participated in a research study.
With respect to the frequencies analyzed in the present study, a threshold value of 0.40 Hz was selected to delineate between low- and high-frequency components of the COP signal (Singer and Mochizuki, 2015), based on previous work (Winter et al., 1998; Zatsiorsky and Duarte, 1999; Zatsiorsky and Duarte, 2000) that described two control mechanisms within models of postural control. The present results provide some support for the validity of these models, as the majority of significant differences between the Failed Response and Successful Response groups were identified at frequencies above 0.40 Hz. Future work should seek to confirm this value as the most appropriate for a cut-off level. In addition, it has been suggested that the majority of the power in the COP spectrum lies within a range of DC to between 1.00–1.40 Hz, depending on the measurement methodology (Vieira et al., 2009). Therefore, the highest frequency range analyzed (1.60–3.20 Hz), although statistically different between groups, may have limited practical significance. The two remaining frequency ranges showing significant differences between groups (0.40–0.80 Hz, 0.80–1.60 Hz) were completely or mostly within the COP power spectrum, and therefore are likely representative of the true COP signal.
4.1 Conclusions
In conclusion, this study identified differences in the reactive control of quiet standing in individuals with stroke based on ability to recover balance. The findings partially supported the hypothesis, in that the individuals with failed responses on the unconstrained lean-and-release trials demonstrated greater RMS of COP in higher frequency levels of the COP signal, specifically in the 0.40–3.20 Hz range. Therefore, individuals with decreased ability to recover balance may also demonstrate reduced postural stability and impaired reactive control of quiet standing. These results provide insight into the mechanism by which the reactive control of quiet standing is impaired in individuals with stroke, and may inform strategies to more effectively assess and rehabilitate reactive balance control during stroke recovery.
Highlights.
Standing centre of pressure signals from individuals with stroke were decomposed.
Decomposed measures were compared based on balance recovery ability.
Individuals with failed balance recovery responses had impaired standing control.
Results provide insight into mechanisms of balance impairment post-stroke.
This technique may be an alternative method to assess balance recovery post-stroke.
Acknowledgments
Funding
Equipment and space have been funded with grants from the Canada Foundation for Innovation, Ontario Innovation Trust, and the Ministry of Research and Innovation. Alison Schinkel-Ivy is supported by a Trainee Award from the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery. Avril Mansfield is supported by a New Investigator Award from the Canadian Institutes of Health Research (MSH-141983). The views expressed do not necessarily reflect those of the funders, and the funders had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
The authors wish to acknowledge the undergraduate co-operative students and the staff of the Balance, Mobility & Falls Clinic at the Toronto Rehabilitation Institute who aided in data collection.
Abbreviations
- CMSA
Chedoke-McMaster Stroke Assessment
- COM
Centre of mass
- COP
Centre of pressure
- NIHSS
National Institutes of Health Stroke Scale (NIHSS)
- RMS
Root mean square
Footnotes
Conflict of Interest
None.
References
- Addison PS. Wavelet transforms and the ECG: A review. Physiol Meas. 2005;26(5):R155–R99. doi: 10.1088/0967-3334/26/5/R01. [DOI] [PubMed] [Google Scholar]
- Batchelor FA, Mackintosh SF, Said CM, Hill KD. Falls after stroke. Int J Stroke. 2012;7(6):482–90. doi: 10.1111/j.1747-4949.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Murnaghan CD, Inglis JT. Shifting the balance: evidence of an exploratory role for postural sway. Neuroscience. 2010;171:196–204. doi: 10.1016/j.neuroscience.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Chagdes JR, Rietdyk S, Haddad JM, Zelaznik HN, Raman A, Rhea CK, et al. Multiple timescales in postural dynamics associated with vision and a secondary task are revealed by wavelet analysis. Exp Brain Res. 2009;197:297–310. doi: 10.1007/s00221-009-1915-1. [DOI] [PubMed] [Google Scholar]
- Clark RA, Howells B, Pua Y-H, Feller J, Whitehead T, Webster KE. Assessment of standing balance deficits in people who have undergone anterior cruciate ligament reconstruction using traditional and modern analysis methods. J Biomech. 2014;47:1134–7. doi: 10.1016/j.jbiomech.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Fryzlewicz P. Wavelet methods. Wiley Interdiscip Rev Comput Stat. 2010;2(6):654–67. [Google Scholar]
- Fujimoto H, Mihara M, Hattori N, Hatakenaka M, Kawano T, Yagura H, et al. Cortical changes underlying balance recovery in patients with hemiplegic stroke. Neuroimage. 2014;85:547–54. doi: 10.1016/j.neuroimage.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Geurts ACH, de Haart M, van Nes IJW, Duysens J. A review of standing balance recovery from stroke. Gait Posture. 2005;22:267–81. doi: 10.1016/j.gaitpost.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Graafmans WC, Oomes ME, Hofstee HMA, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143:1129–36. doi: 10.1093/oxfordjournals.aje.a008690. [DOI] [PubMed] [Google Scholar]
- Hendrickson J, Patterson KK, Inness EL, McIlroy WE, Mansfield A. Relationship between asymmetry of quiet standing balance control and walking post-stroke. Gait Posture. 2014;39(1):177–81. doi: 10.1016/j.gaitpost.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Holliday PJ, Fernie GR, Gryfe CI, Griggs GT. Video recording of spontaneous falls of the elderly. In: Gray BE, editor. Slips, Stumbles, and Falls: Pedestrian Footwear and Surfaces. Philadelphia: American Society for Testing and Materials; 1990. pp. 7–16. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
- Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83:165–70. doi: 10.1053/apmr.2002.28030. [DOI] [PubMed] [Google Scholar]
- Inness EL, Mansfield A, Biasin L, Brunton K, Bayley M, McIlroy WE. Clinical implementation of a reactive balance control assessment in a sub-acute stroke patient population using a ‘lean-and-release’ methodology. Gait Posture. 2015;41:529–34. doi: 10.1016/j.gaitpost.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Inness EL, Mansfield A, Lakhani B, Bayley M, McIlroy WE. Impaired reactive stepping among patients ready for discharge from inpatient stroke rehabilitation. Phys Ther. 2014;94:1755–64. doi: 10.2522/ptj.20130603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–48. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen L, Engstad T, Jacobson BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. 2002;33:542–7. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- Kiemel T, Oie KS, Jeka JJ. Slow dynamics of postural sway are in the feedback loop. J Neurophysiol. 2006;95:1410–8. doi: 10.1152/jn.01144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:1–12. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani B, Mansfield A, Inness EL, McIlroy WE. Compensatory stepping responses in individuals with stroke: a pilot study. Physiother Theory Pract. 2011;27(4):299–309. doi: 10.3109/09593985.2010.501848. [DOI] [PubMed] [Google Scholar]
- Latash ML, Ferreira SS, Wieczorek SA, Duarte M. Movement sway: Changes in postural sway during voluntary shifts of the center of pressure. Exp Brain Res. 2003;150:314–24. doi: 10.1007/s00221-003-1419-3. [DOI] [PubMed] [Google Scholar]
- Mackey DC, Robinovitch SN. Postural steadiness during quiet stance does not associate with ability to recover balance in older women. Clin Biomech. 2005;20(8):776–83. doi: 10.1016/j.clinbiomech.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Fernie GR. Aging and postural control: a comparison of spontaneous- and induced-sway balance tests. J Am Geriatr Soc. 1990;38:1–9. doi: 10.1111/j.1532-5415.1990.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–M84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Postural control in the older adult. Clin Geriatr Med. 1996;12(4):635–58. [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Phys Ther. 1997;77:488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Change-in-support balance reactions in older persons: an emerging research area of clinical importance. Neurol Clin. 2005;23(3):751–83. doi: 10.1016/j.ncl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE, Fernie GR. Change-in-support reactions for balance recovery: control mechanisms, age-related changes and implications for fall prevention. IEEE Eng Med Biol Mag. 2003;22(2):20–6. doi: 10.1109/memb.2003.1195691. [DOI] [PubMed] [Google Scholar]
- Mansfield A, Danells CJ, Inness EL, Mochizuki G, McIlroy WE. Between-limb synchronization for control of standing balance in individuals with stroke. Clin Biomech. 2011;26(3):312–7. doi: 10.1016/j.clinbiomech.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil Neural Repair. 2013;27(6):526–33. doi: 10.1177/1545968313478486. [DOI] [PubMed] [Google Scholar]
- Mansfield A, Mochizuki G, Inness EL, McIlroy WE. Clinical correlates of between-limb synchronization of standing balance control and falls during inpatient stroke rehabilitation. Neurorehabil Neural Repair. 2012;26(6):627–35. doi: 10.1177/1545968311429688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield A, Wong JS, McIlroy WE, Biasin L, Brunton K, Bayley M, et al. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy. 2015;101(4):373–80. doi: 10.1016/j.physio.2015.01.009. [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech. 1997;12(1):66–70. doi: 10.1016/s0268-0033(96)00040-x. [DOI] [PubMed] [Google Scholar]
- Mochizuki G, Boe S, Marlin A, McIlroy WE. Perturbation-evoked cortical activity reflects both the context and consequence of postural instability. Neuroscience. 2010;170:599–609. doi: 10.1016/j.neuroscience.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Mochizuki G, Sibley KM, Esposito JG, Camilleri JM, McIlroy WE. Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clinical Neurophysiology. 2008;119:1626–37. doi: 10.1016/j.clinph.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Morales CJ, Kolaczyk ED. Wavelet-based multifractal analysis of human balance. Ann Biomed Eng. 2002;30:588–97. doi: 10.1114/1.1478082. [DOI] [PubMed] [Google Scholar]
- O’Loughlin JL, Robitaille Y, Boivin J-F, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–54. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- Owings TM, Pavol MJ, Foley KT, Grabiner MD. Measures of postural stability are not predictors of recovery from large postural disturbances in healthy older adults. J Am Geriatr Soc. 2000;48:42–50. doi: 10.1111/j.1532-5415.2000.tb03027.x. [DOI] [PubMed] [Google Scholar]
- Paillex R, So A. Posture debout chez sujet adultes: Spécificités de l’hémiplégie. Ann Readapt Med Phys. 2003;46:71–8. doi: 10.1016/s0168-6054(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Parokaran Varghese J, Beyer KB, Williams L, Miyasike-daSilva V, McIlroy WE. Standing still: Is there a role for the cortex? Neurosci Lett. 2015;590:18–23. doi: 10.1016/j.neulet.2015.01.055. [DOI] [PubMed] [Google Scholar]
- Prosser LA, Lee SCK, Barbe MF, VanSant AF, Lauer RT. Trunk and hip muscle activity in early walkers with and without cerebral palsy - A frequency analysis. J Electromyogr Kinesiol. 2010;20:851–9. doi: 10.1016/j.jelekin.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioul O, Vetterli M. Wavelets and signal processing. IEEE Signal Process Mag. 1991;8(4):14–38. [Google Scholar]
- Singer JC, Mochizuki G. Post-stroke lower limb spasticity alters the interlimb temporal synchonisation of centre of pressure displacements across multiple timescales. IEEE Trans Neural Syst Rehabil Eng. 2015;23(5):786–95. doi: 10.1109/TNSRE.2014.2353636. [DOI] [PubMed] [Google Scholar]
- Suarez H, Sotta G, San Roman C, Arocena S, Ferreira E, Geisinger D, et al. Postural response characterization in elderly patients with bilateral vestibular hypofunction. Acta Otolaryngol. 2013;133:361–7. doi: 10.3109/00016489.2012.739731. [DOI] [PubMed] [Google Scholar]
- Vieira TMM, Oliveira LF, Nadal J. Estimation procedures affect the center of pressure frequency analysis. Braz J Med Biol Res. 2009;42:665–73. doi: 10.1590/s0100-879x2009000700012. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, de Niet M, van Duijhoven HJR, Geurts ACH. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195–213. [PubMed] [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80(3):1211–21. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurobiol. 1996;75(6):2334–43. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Stergiou P, Powell C. Medial-lateral and anterior-posterior motor responses associated with centre of pressure changes in quiet stance. Neurosci Res Commun. 1993;12(3):141–8. [Google Scholar]
- Zatsiorsky VM, Duarte M. Instant equilibrium point and its migration in standing tasks: Rambling and trembling components of the stabilogram. Motor Control. 1999;3:28–38. doi: 10.1123/mcj.3.1.28. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Duarte M. Rambling and trembling in quiet standing. Motor Control. 2000;4:185–200. doi: 10.1123/mcj.4.2.185. [DOI] [PubMed] [Google Scholar]