Table 2.
Structure–Reactivity Relationship Studies of Nucleophilic Fluorination Reagents
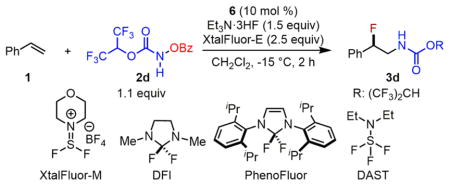
| ||
|---|---|---|
| entrya | variation from the std conditions | observations |
| 1 | replace Et3N·3HF with Et3N·HF, Et3N·2HF, TBAF(tBuOH)4, or AgF | decomposition of 2d, no desired product |
| 2 | replace XtalFluor-E with XtalFluor-M | 3d (65%) with full conversion |
| 3 | replace Et3N·3HF/XtalFluor-E with benzoyl fluoride | 3d (<5%) with 66% conversionb |
| 4 | replace Et3N·3HF/XtalFluor-E with DFI or PhenoFluor | decomposition of 2d, no desired product |
| 5 | replace Et3N·3HF/XtalFluor-E with DAST | decomposition of 2d, no desired product |
| 6 | replace Et3N·3HF/XtalFluor-E (1.5 equiv/2.5 equiv) with DAST/XtalFluor-E (1.5 equiv/ 1.0 equiv) | 3d (51%) with full conversion |
Reactions were carried out under N2 at −15 °C in 2 h, and 4 Å activated molecular sieves (powder) were used to remove deleterious moisture, unless stated otherwise.
Amino-oxygenation product 4d (42%) and benzoylated 2d (24%) were isolated.
