Abstract
Background
High grade gliomas are associated with a dismal prognosis. Notch inhibition via the gamma-secretase inhibitor RO2929097 has emerged as a potential therapeutic option based on modulation of the cancer-initiating cell (CIS) population and a presumed anti-angiogenic role.
Methods
In this phase 0/I trial, 21 patients with newly-diagnosed glioblastoma or anaplastic astrocytoma received RO4929097 combined with temozolomide and radiotherapy. In addition to establishing the maximum tolerated dose, the study design enabled exploratory studies evaluating tumor and brain drug penetration and neuro-imaging parameters. We also determined functional effects on the Notch pathway and targeting of CISs through analysis of tumor tissue sampled from areas with and without blood-brain barrier disruption. Finally, recurrent tumors were also sampled and assessed for Notch pathway responses while on treatment.
Results
Treatment was well tolerated and no dose-limiting toxicities were observed. Immunohistochemistry of treated tumors showed a significant decrease in proliferation and in the expression of the Notch intracellular domain (NICD) by tumor cells and blood vessels. Patient-specific organotypic tumor explants cultures revealed a specific decrease in the CD133+ CIS population upon treatment. Perfusion MRI demonstrated a significant decrease in relative plasma volume after drug exposure. Gene expression data in recurrent tumors suggested low Notch signaling activity, the upregulation of key mesenchymal genes and an increase in VEGF-dependent angiogenic factors.
Conclusion
The addition of RO4929097 to temozolomide and radiotherapy was well tolerated; the drug has variable blood-brain barrier penetration. Evidence of target modulation was observed, but recurrence occurred, associated with alterations in angiogenesis signaling pathways.
Keywords: Notch Inhibition, Gamma-Secretase Inhibitor, Anaplastic Astrocytoma, Glioblastoma, High grade glioma, Phase I Trial, Cancer-Initiating Cells
Introduction
High grade gliomas, including glioblastoma and WHO grade III anaplastic astrocytomas (AA), are the most common and aggressive primary brain tumor in adults. Despite multidisciplinary approaches that combine maximal surgical resection and radiotherapy (RT) with concomitant and adjuvant temozolomide, glioblastomas remain associated with a dismal prognosis, with clinical trials in newly-diagnosed disease reporting median overall survival (OS) of 15–18 months (1–3).
Recent literature supports a role for glioma stem cells (GSC), which represent a subfraction of the tumor cell pool and are thought to be crucial to tumor growth (4). The Notch signaling pathway plays a key role during the development of the central nervous system, including maintenance of self-renewing neural progenitors and regulation of their fate decisions by promoting glial differentiation (5). Notch signaling has also been implicated in cancer with putative roles in tumor stem cell biology, angiogenesis, and tumor progression (6–8). The four transmembrane Notch receptors (Notch 1–4) interact closely with membrane-bound ligands that belong to the Jagged (Jagged ligands 1 and 2) or Delta-like family (DLL 1, 3, and 4) (9). Upon ligand-receptor binding, the Notch receptor undergoes proteolytic cleavage by metalloprotease and gamma-secretase, which releases the Notch intracellular domain (NICD) that translocates to the nucleus and activates target gene transcription (10).
Our laboratory and others have investigated the role of Notch inhibition in the GSC population (11–14). Using 3D organotypic tumor explants, we demonstrated Notch inhibition via a gamma-secretase inhibitor (GSI) is associated with a decrease in GSCs, as well as a decrease in endothelial cells (11). We also demonstrated that combination treatment of tumor explants with Notch inhibition and radiation was more effective than either treatment alone, suggesting a synergistic effect (11, 15). These findings formed the scientific rationale for the design of the present trial.
Given its significant role in tumor biology, in vivo Notch pathway modulation has been studied with a range of inhibitors, including GSIs, other small molecule inhibitors as well as targeted monoclonal antibodies (16, 17). RO4929097 is an orally bioavailable small molecule GSI, capable of a potent, inhibitory effect on Notch signaling (17, 18). It has been evaluated in early phase trials in solid tumors, alone or in combination with other agents (19–24), with responses observed in a range of tumor types. This early experience also raised some concerns about drug-drug interactions and auto-induction, and identified GSI-associated toxicities. Of note is a phase I trial of 103 patients with advanced solid tumors conducted with GSI MK-0742, which reported a complete response in one AA and stable disease in 10 patients with glioblastoma (25).
Treatment of brain tumors is often limited by the ability of small molecules to cross the blood brain barrier (BBB) and achieve therapeutic concentrations in tumor tissue. There are currently no data pertaining to BBB permeability of RO4929097 or its effectiveness in achieving inhibition of Notch signaling in brain tumors upon systemic administration.
Here we report a phase 0/I study of RO4929097 in combination with TMZ and radiation therapy in patients with newly diagnosed glioblastoma or WHO grade III AA. The primary goals were to determine the maximum-tolerated dose (MTD), toxicities and pharmacokinetic (PK) effects. Secondary goals of this proof-of-concept study included exploratory analyses of tumor drug penetration, evaluation of patients’ GSC populations and in vivo Notch target modulation, as well as the evaluation of drug effects on advanced neuro-imaging, including dynamic contrast-enhanced (DCE) perfusion MRI. The trial also included analyses of tissue samples obtained intraoperatively and grown in 3D organotypic cultures as a means of evaluating the drug effects on tumor tissue ex-vivo, following a clinical-laboratory co-development paradigm. We also had the opportunity of sampling recurrent tumors while on treatment, thus establishing a comparative profile of gene expression.
Materials and Methods
Study Design
Patients with newly-diagnosed glioblastoma or AA who had undergone biopsy or partial resections prior to enrollment, and that upon further neurosurgical evaluation had an indication for additional debulking surgery were eligible for this study; anaplastic oligodendroglioma or 1p/19q co-deleted tumors were excluded. Other standard inclusion and exclusion criteria are detailed in Supplemental Methods.
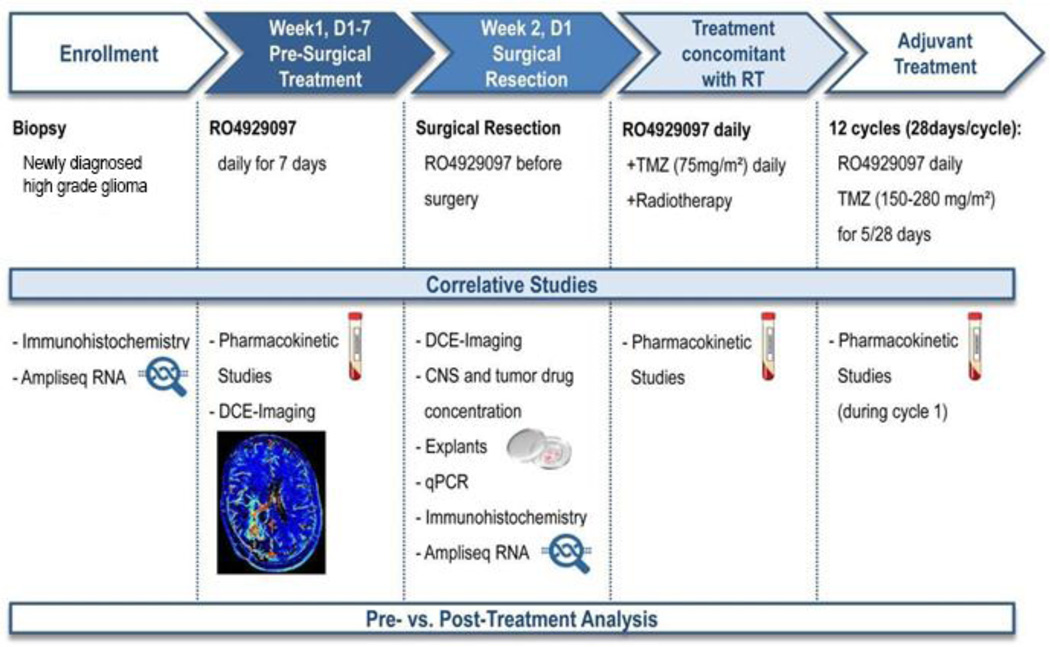
The study design is summarized in Figure 1 and Supplemental Figure S1. Upon establishment of date of surgery, patients received RO4929097 daily for 7 days, followed by surgical resection on day 7. The last dose was administered 2–3 hours prior to resection, at which time tissue and blood samples were obtained for correlative studies. Following surgery, RO4929097 was discontinued to allow for recovery and wound healing.
Figure 1. Study design and treatment plan for enrolled patients.
Patients with newly diagnosed glioblastoma or anaplastic astrocytoma were treated with RO4929097 (in escalating doses/3+3 phase I design) for 7 days, followed by surgical resection, radiotherapy (RT) and temozolomide (TMZ) administration. Pharmacokinetic studies were performed on plasma and tumor tissue; surgical samples were also used for histochemical and molecular studies.
A phase I 3+3 design was then applied to evaluate the combination of RT, temozolomide and RO4929097. Patients with adequate performance status after surgery were re-started on daily RO4929097 and received standard RT concomitant with temozolomide 75mg/m2 daily, followed by adjuvant temozolomide given at a dose of 150–200mg/m2 days 1–5 of 28-day cycles, for a minimum of 6 and a maximum of 12 cycles. Standard 3+3 dose escalation rules were utilized, with dose-liming toxicities based on adverse events occurring in the first 30 days of RT.
Three pre-specified dose levels of RO4929097 were examined (10mg, 15mg and 20mg once a day), selected based on available PK studies of RO4929097 in solid tumors that indicated doses above 20mg do not increase drug exposure due to auto-induction.
To avoid delays in establishing the maximum tolerated dose (MTD), patients who were not candidates for post-operative treatment due to poor performance status or co-morbidities were replaced by patients already resected off-study (n=11). Enrollment of additional patients who were surgical candidates was permitted while a given cohort was being examined, for a maximum of 10 additional patients.
Tumor status (MacDonald criteria) was assessed every two months. Patients with suspected pseudoprogression were allowed to stay on treatment if deemed appropriate by the treating physician, or offered surgical resection. If progression was confirmed on next scan or histologically, patients were removed from study and the date of first MRI showing radiographic progression was considered the date of progression. If further tumor resections were indicated during the study, i.e. in patients with suspicion of tumor progression or pseudo-progression, patients were asked to remain on RO4929097 until day of surgery, during which tumor tissue samples were also collected for further comparative analysis (n=7).
Exploratory Neuro-Imaging Evaluation
To explore early pharmacodynamic effects via changes in advanced neuro-imaging parameters, notably perfusion and vascular permeability, patients undergoing surgical resection were evaluated with DCE-MRI at baseline and then again after 7 days of RO4929097 (i.e. immediately before surgery) (Supplemental methods). Analyses focused on blood perfusion as measured by relative fractional plasma volume (Vp) and vascular permeability as measured by volume transfer coefficient between plasma and extravascular extracellular space (Ktrans).
Pharmacokinetic Studies
High-performance liquid chromatography with tandem mass spectrometry was utilized to determine levels of RO4929097 in blood and tumor tissue. Tumor areas with and without contrast-enhancement, as defined by intraoperative MRI, were differentially sampled. Additionally, standard blood PK parameters of RO4929097 were evaluated in combination with TMZ 75mg/m2 and 150mg/m2 (More detail in Supplemental Methods).
Quantitative Real-Time PCR, Immunohistochemistry, Morphometry and RNA Sequencing
Methods utilized for evaluation of effects of RO4929097 on gene expression, Notch pathway, angiogenesis and GSC are detailed in Supplement methods. Archival and on-study surgery FFPE tumor tissue was utilized for quantitative real-time PCR and RNA sequencing. Immunohistochemistry was performed utilizing anti-NICD and anti-CD31 antibodies. Morphometry was performed using ImageJ.
Viability and Flow Cytometry Analysis of Organotypic Explants
In addition to the analyses above, fresh tumor tissue from patients operated on study were grown as 3-D organotypic explants for further characterization of in vitro effects of Notch inhibition (20, 45) (Supplement methods). Established explants were treated with RT with TMZ, or combined RT, TMZ and RO4929097, or single agent RO4929097, then evaluated for effects on cell proliferation (Ki-67 immunofluorescent staining), tumor cell viability (CellTiter Glo Luminescent Assay) and proportion of GSC-like CD133+ cells (FACS analysis).
Statistical Analysis
All values are expressed as mean±SEM unless otherwise stated. Statistical significance was determined by unpaired Student's t-tests. Survival was calculated from date of initial histologic diagnosis to either death (OS), or progression (PFS) utilizing Kaplan-Meier methodology (Stata 9.3). Patients were censored at last follow-up.
Study Approval
This study was an investigator-initiated, prospective phase 0/I clinical trial (ClinicalTrials.gov identifier: NCT01119599), primarily sponsored by the NCI/ Cancer Treatment and Evaluation Program (CTEP); NCI# 8556. The study was approved by the MSKCC Institutional Review Board. Written informed consent was obtained from all participants or guardians prior to enrollment.
Results
Study Population
Twenty-one patients with newly-diagnosed glioblastoma (n=17) or WHO grade III AA (n=4) were enrolled between 5/2010–6/2012. Median age was 57 years (range: 28–68) and median KPS was 90. Patient characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of patients enrolled in the study
| Characteristic | All patients | GBM | AA |
|---|---|---|---|
| Number of patients enrolled |
21 | 17 | 4 |
| Median age | 57 | 58 | 38 |
| range | 28 – 68 | 36 – 65 | 28 – 68 |
| Gender | |||
| Women | 7 | 6 | 1 |
| Men | 14 | 11 | 3 |
| Median KPS | 90 | 90 | 95 |
| range | 80 – 100 | 80 – 100 | 90 – 100 |
| RO4929097 dosage | |||
| 10 mg | 4 | 3 | 1 |
| 15 mg | 7 | 6 | 1 |
| 20 mg | 10 | 8 | 2 |
| Surgical candidates* | 10 | 10 | 0 |
| Replacement patients* | 11 | 7 | 4 |
| MGMT promoter | |||
| methylated | 4 | 4 | 0 |
| unmethylated | 12 | 11 | 1 |
| undetermined | 5 | 2 | 3 |
| IDH-1 R132H mutation | |||
| present | 2 | 0 | 1 |
| absent | 5 | 5 | 1** |
| not tested | 16 | 12 | 2 |
Patients who were candidates for surgical resection were primarily enrolled. Patients who were not candidates for a clinical trial after surgery were replaced by patients with tumor already resected off trial.
This patient had an IDH-1 R132C mutation on next generation sequencing. MGMT promoter status determined by methylation-specific PCR. GBM: Glioblastoma; AA: Anaplastic astrocytoma.
All three pre-specified RO4929097 dose levels were examined: 10mg (N=4), 15mg (N=7) and 20mg (N=10). Seven patients required repeat surgical resection for tumor progression while on RO4929097 and TMZ, and had tissue analyzed for comparative analyses.
Treatment, Dose Escalation and Toxicity
Treatment was well tolerated, and dose escalation proceeded as planned. No dose-limiting toxicities were observed in any of the pre-specified dose levels. The 20mg daily dose was deemed the recommended phase 2 dose (RP2D). A summary of all reported grades 3 and 4 adverse events is shown in Supplemental Table 1. Toxicities were manageable or reversible; no patient discontinued treatment due to toxicities, and there were no treatment-related deaths. The toxicity profile is comparable to previously reported profiles for temozolomide and RO4929097, consisting mainly of hematologic toxicities and elevated transaminases. The relationship between electrolyte disturbances and treatment is uncertain, but such events were conservatively designated as possibly related to treatment. Other unrelated or unlikely related adverse events, inherent to brain tumors (e.g. intra-cranial hemorrhage, complications from surgery, neurological symptoms, thromboembolic events and hyperglycemia in the setting of corticosteroids), were observed as expected. One patient developed retroperitoneal bleeding in the setting of a mechanical fall and use of aspirin. There was no significant QTc prolongation.
Pharmacokinetic Evaluation
To evaluate effects of temozolomide on the plasma PK parameters of RO4929097, standard 24-hour PK evaluations were performed during temozolomide 75mg/m2 (first day of treatment and after 5 weeks) and during first day of adjuvant temozolomide 150 mg/m2/day. Mean (±SD) values of maximum plasma concentration (Cmax) and the area under the plasma concentration-time curve for the 24h dosing interval (AUC24) across the different RO4929097 dose levels are presented in Supplemental Table 2. The mean Cmax and AUC24 for the initial dose of RO4929097 increased as the dose was escalated and values were consistent with previously reported clinical studies of single–agent RO4929097 (19, 23). In addition, the mean Cmax and AUC24 values determined after 10 weeks of continuous daily dosing on day 1 of adjuvant therapy were lower than the corresponding values for the initial dose at each dose level, consistent with the previously reported auto-induction of RO4929097 elimination.
Effect of RO4929097 on MRI Perfusion Parameters
Eight patients underwent the exploratory pharmacodynamic evaluation with DCE-perfusion MRI obtained before and after 7 days of exposure to RO4929097 (Supplemental Figure 2A). All patients demonstrated a decrease in perfusion, with mean Vp ratio decreasing from 9.34± 2.4 at baseline to 3.92± 0.9 after drug exposure (p<0.05) (Supplemental Figure S2B). Likewise, there was a trend towards a decrease in vascular permeability, with a mean Ktrans of 0.05± 0.01 min−1 at baseline and 0.03± 0.01 min−1 after treatment (Supplemental Figure S2C). These findings suggest an early attenuating effect on tumor blood perfusion following exposure to RO4929097.
Brain and Tumor Penetration of RO4929097
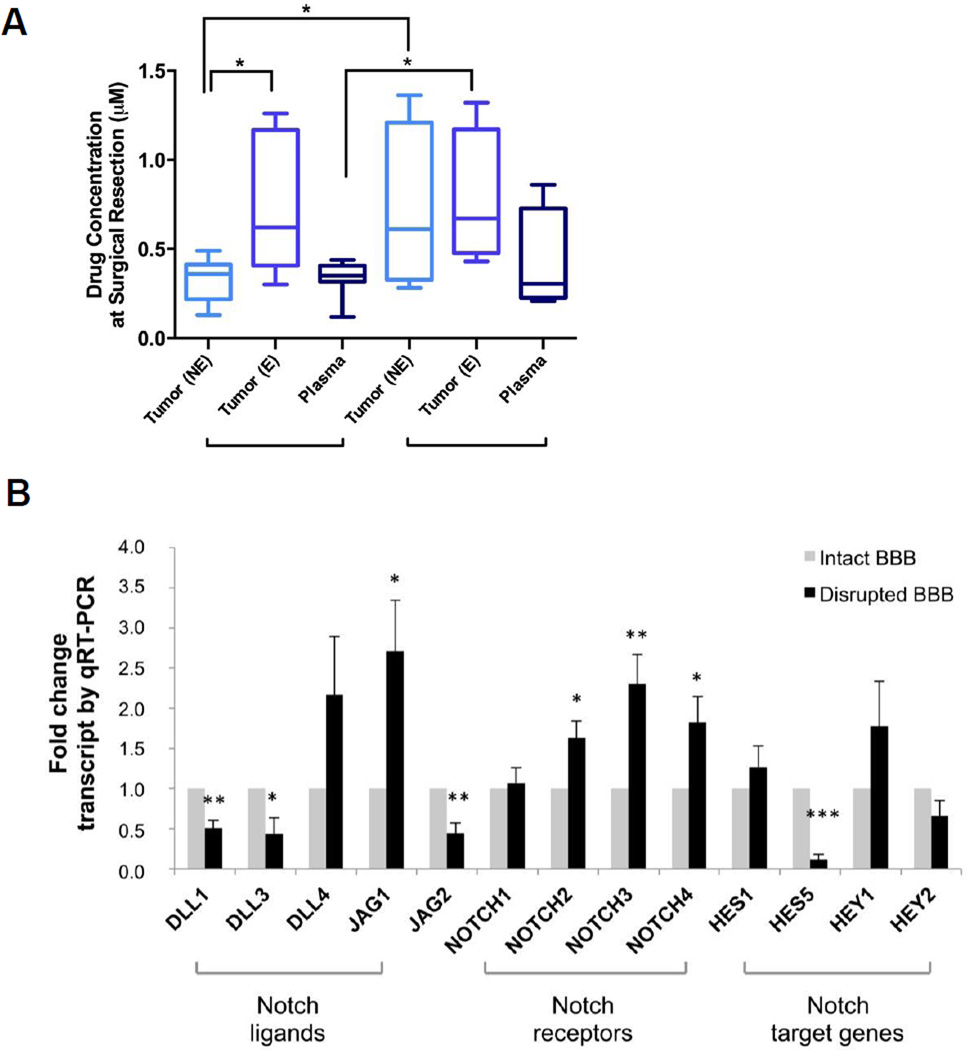
Intraoperative biopsy of contrast enhancing and non-enhancing tumor tissue and concomitant acquisition of plasma samples were obtained in 11 patients. Areas of enhancement on T1 post-gadolinium MRI represent areas of BBB breakdown and increased permeability, whereas areas without contrast enhancement have a relatively intact BBB. It has been posited that tumor cells in areas of intact BBB (no enhancement) are often not exposed to adequate drug levels, contributing to the lack of efficacy of treatments for glioma. Our data show drug levels upon initial administration of RO4929097 (i.e. 7 days culminating in surgery) were significantly higher in enhancing (0.73 ± 0.13 µM) versus non-enhancing (0.33 ± 0.04 µM) tissue (Figure 2A). Available literature demonstrate a reduction in target genes (e.g. Hes1) with RO4929097 in a dose-dependent manner starting at 0.1 µM (24), thus suggesting the achieved drug levels were within therapeutic levels regardless of BBB integrity. Interestingly, in patients who presented with tumor progression that required a second surgery after a prolonged course of RO4929097 (mean: 181 days, range: 104 – 399 days), tumor drug concentrations were similar in enhancing and non-enhancing areas, reaching an average of 0.77 ± 0.19 µM and 0.71 ± 0.24 µM respectively (Figure 2A).
Figure 2. Drug concentration levels and mRNA expression profile in enhancing and non-enhancing brain tissue.
A, Tumor drug concentration levels (ng/mg) after 7-day treatment (n=8) and prolonged RO-treatment (n=4) are shown in areas of tumor tissue with intact (non-enhancing) and disrupted (enhancing) BBB, as distinguished by T1-weighted Gadolinium-enhanced MRI. B, mRNA expression profile in enhancing and non-enhancing brain tumor tissue of Notch pathway components; levels are normalized to 18S (n=6). Values are expressed as mean ± S.E.M. (BBB = blood brain barrier, E = enhancing; NE = non-enhancing; *p<0.05, **p<0.01, ***p<0.001).
To assess the extent to which drug concentrations in the blood influenced intratumoral drug concentrations, the corresponding ratios of tumor-to-plasma molar drug concentrations were determined at the time of surgery (following 7 days of RO4929097 administration), and were found to be approximately 1 in the non-enhancing areas, but twice as high in the areas of BBB breakdown and enhancement (Figure 2 and Supplemental Table 3). Upon repeat surgery for recurrence in the setting of prolonged RO4929097 use, drug accumulated in areas of presumed normal (non-enhancing) and disrupted BBB (enhancing), leading to tumor-to-plasma ratios that are similar to those measured in the enhancing regions after 7 days of treatment, averaging 2.7 in enhancing and 2.5 in non-enhancing brain tissue. However, the range of concentrations exhibited significant variability with one specimen (M09) reaching levels of ~1.3 µM in non-enhancing tumor tissue (Supplemental Table 3). A notable distinction is that patients with a prolonged RO4929097 course had also received radiation treatment (average time since RT: 131 days [range: 57 – 357 days]).
We further dissected the potential impact of RO4929097 treatment on Notch pathway components, using tumor tissue obtained from both areas displaying BBB breakdown (contrast-enhancing) and intact BBB (non-contrast-enhancing). Intraoperative tumor specimens were processed for quantitative mRNA analysis using a panel of primers against a range of Notch ligands, receptors and gene targets. In comparison to areas of intact BBB, enhancing tissue showed significant downregulation of DLL1, DLL3 and Jag2 ligands, as well as suppression of the downstream effector Hes5, whereas Hes1 levels remained unchanged (Figure 2B). Interestingly, there was also significant upregulation of several Notch receptors (Notch 2, 3 and 4), which may suggest activation of negative feedback loops due to the suppression of Hes5.
Effect of RO4929097 on Proliferation, the Notch pathway and Tumor Blood Vessels
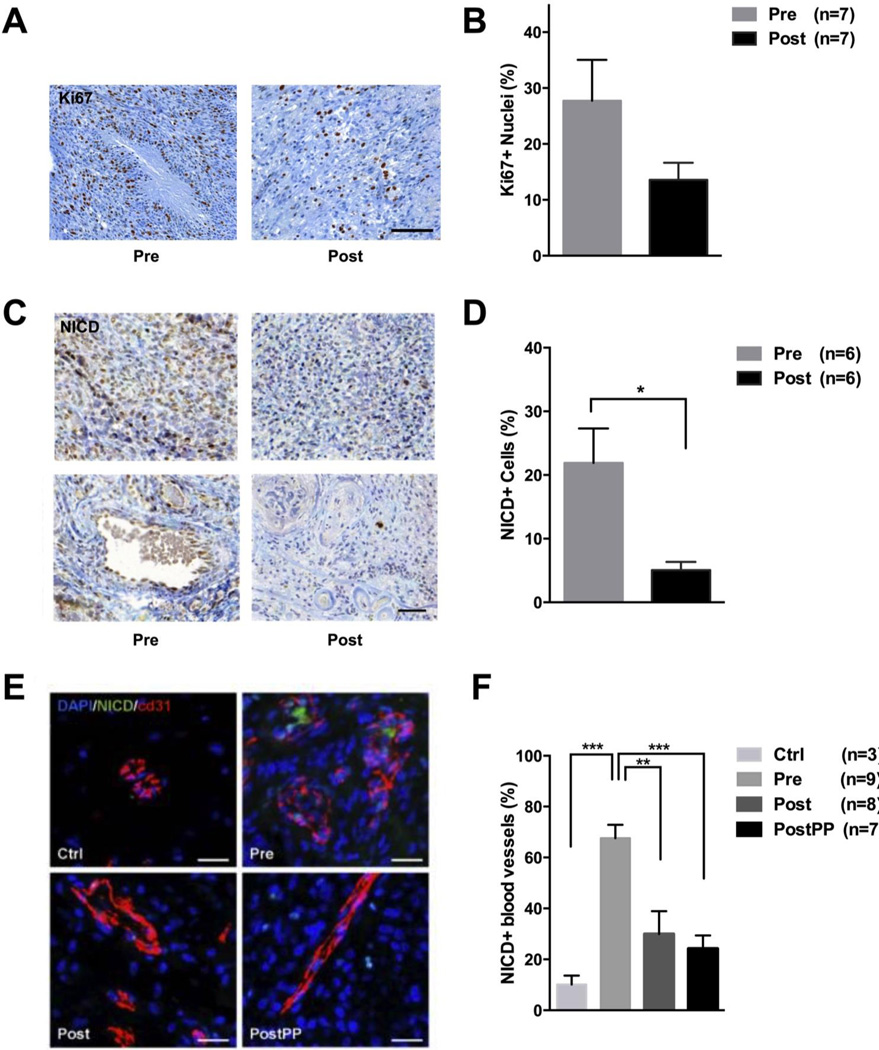
We next sought to validate whether the drug levels achieved in tumor tissue were sufficient to impact tumor cells proliferation. Immunohistochemical analyses of the Ki-67 proliferation marker in matching tumor samples, obtained before and after 7 days of RO treatment, showed a trend towards a decrease in proliferation from 27.7 ± 7.3 % to 13.6 ± 3.1 %, albeit with marked inter-patient variability (Figures 3A–B and Supplemental Figure S3).
Figure 3. Post-treatment effects of RO4929097 on proliferation, Notch receptor and surrounding blood vessels.
A, Immunohistochemistry (IHC) and quantification of Ki-67 labeling index in matched pre- and post-treatment tumor tissue, before and after treatment (n=7). C, NICD (nuclear intracellular domain of Notch) IHC of matched pre- and post-treatment glioblastoma patient samples. Lower panel shows NICD-positive cells in relation to vascularity. D, Quantification of NICD+ tumor cells after 7 days of RO4929097 treatment (n=6). E, Sections from normal brain (Ctrl), enrolled glioblastoma patients before (Pre), after 7 days (Post) and after multiple cycles (PostPP) of RO4929097 treatment were immunostained for NICD and vessel marker CD31. F, Quantification of NICD+ vascular structures as percent of total blood vessels in sections of normal brain tissue and glioblastoma samples before and after RO4929097 exposure. A minimum of 1,000 cells were counted for Ki-67 and NICD analysis; the entire tissue area was quantified to determine the ratio of NICD+ vascular structures. Values are expressed as mean ± S.E.M. (*p<0.05, **p<0.01, ***p<0.001).
RO4929097 potently inhibits the enzymatic cleavage event that the Notch receptor undergoes upon its activation, followed by release of NICD and its translocation to the nucleus (24). Therefore, we analyzed the expression of NICD in tumor cells in matching pre- and post-treatment glioblastoma tissue, via immunohistochemistry. The number of NICD-positive cells decreased significantly from 21.9 ± 5.4 % to 5.1 ± 1.3 % after the initial presurgical 7-day treatment with RO4929097 (Figure 3C–D), suggesting a state of Notch signaling inactivity or inhibition. Moreover, a notable difference in NICD expression was observed within tumor vasculature (Figure 3C, lower panel). These data are suggestive of biological activity of RO4929097 within tumor tissue.
Notch activity plays an important role in tumor angiogenesis but the role of Notch inhibition on glioma vascularity remains unclear (33–35). We therefore sought to determine the effect of RO4929097 treatment on glioma vessels through analysis of available matching pre- and post-treatment tissues. Immunohistochemical evaluation of tissues obtained before treatment (Pre), at 7 days of RO4929097 (Post) and at the time of recurrence after prolonged treatment (PostPP), shows no significant change in microvascular density, but a significant decrease in NICD-expressing vascular structures from 67.6 ± 5.2 % (Pre) to 30.0 ± 9.0 % (Post) and 24.2 ± 5.2 % (PostPP) in matching samples (Figure 3E–F). Expression of NICD in normal brain vessels was assessed in a set of normal brain controls and found to represent 10.1 ± 3.5 % of blood vessels (Figure 3E–F). Furthermore, vascular morphology in pre-treatment tissue showed characteristic glomeruloid body formation whereas post-treatment tissue demonstrated a vascular phenotype that was more normalized, with largely monolayers of endothelial cells lining sometimes large but often more normal appearing vascular spaces (Figure 3E). Taken together, these data suggest a modulation of glioma vasculature during RO4929097 treatment, and are consistent with the decreased perfusion observed on DCE-MRI.
Effect of RO4929097 Alone and in Combination with RT and TMZ on the Glioma Stem Cell Population in Organotypic Slices
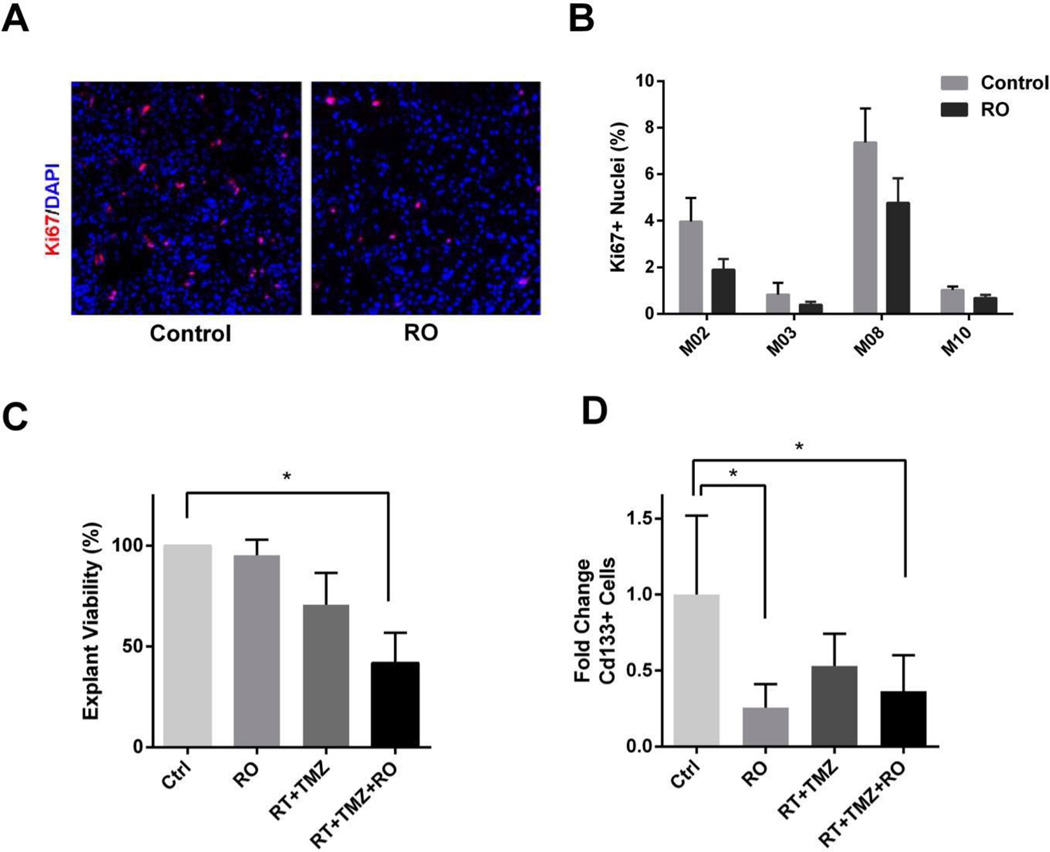
Our group and others have previously shown that inhibition of Notch signaling leads to a decrease in the number of putative CD133+ GSC (11, 26). We have also demonstrated the importance of the vascular microenvironment in effecting the role of Notch inhibition, using tumor “organotypic” explants. Here we established tumor explants from patients in whom adequate fresh tissue could be obtained during surgery (n=4). Explants were treated for 10 days with RO4929097 (1 µM) in vitro, following which there was a significant decrease in proliferation (assessed by Ki-67 quantification) (Figure 4A–B). Having demonstrated in previous studies synergistic effects between radiation and Notch inhibition, we focused on analyzing the impact of RO4929097, RT+temozolomide and RT+temozolomide+RO4929097 on cell viability in tumor explants; the latter condition being representative of the clinical trial treatment conditions (11). Only the triple combination (RT+temozolomide+RO4929097) had a significant attenuating effect on tumor cell viability, effecting a nearly 60% decrease compared to the control untreated explants (Figure 4C). Finally, Notch activation in tumor cells is thought to be mediated by ligand-bearing endothelial cells and is greatest around tumor vasculature, an area thought to represent a stem cell niche (11, 27, 28). We therefore analyzed the proportion of GSC-like CD133+ cells in the explants by FACS analysis. The data show a significant decrease in the proportion of CD133+ cells following treatment with RO4929097 alone (Figure 4D). Interestingly, neither the combination of RT+temozolomide (standard of care) nor the triple combination (RT+temozolomide+RO4929097) were associated with a further reduction in the CD133+ population (Figure 4D).
Figure 4. Effect of RO4929097 treatment on proliferation, viability and CD133+ glioma stem cells in glioblastoma explants.
A, Immunofluorescent staining of Ki-67 in organotypic explants before and after treatment with RO. B, Quantification of Ki-67+ nuclei in patient explants before and after drug treatment (n=4). C, Cell viability in tumor explants treated with RO4929097, RT and TMZ, and the triple combination, as measured by CellTiter Glo Luminescent Assay (n=5). D, FACS-Analysis of cd133+ cells in tumor explants after drug treatment (n=5). (Ctrl = control, RO = RO4929097, RT = radiotherapy, TMZ = temozolomide; *p<0.05).
Gene Expression Analysis post Treatment
Gene expression profiling was performed on a set of matched tissues obtained pre-treatment and after the initial 7 days of drug therapy. In addition, seven patients underwent surgery upon tumor recurrence while on prolonged drug treatment. To dissect gene expression patterns, we expanded our transcriptomal analysis via a customized RNA-Ampliseq panel including glioma lineage markers, Notch pathway components, signaling mediators of angiogenesis and the EGFR pathway (Supplemental Figure S4 A, B). Results showed a trend towards downregulation of the primary Notch gene targets Hes5, Hey1 and Hey2 mostly in the recurrence group with prolonged RO 4929097 therapy, although differences did not reach statistical significance. Gene expression data in the recurrent group (Supplemental Figure S4B) also demonstrated an increase in angiogenesis markers, as well as in C/EBPB (CCAAT/Enhancer Binding Protein Beta) and STAT 3, genes considered master regulators of the mesenchymal phenotype in gliomas. Tumor recurrence in the setting of prolonged RO4929097 therapy therefore occurred in a background of low Notch activity, suggesting an “escape” from Notch dependence. The recurrent tumors exhibited upregulation of angiogenesis markers such as PECAM (CD31), angiopoetins and VEGFA, suggesting a transition to a potential alternative Notch-independent neo-angiogenesis (Supplemental Figure S4B). Interestingly, the mesenchymal-associated genes C/EBPB and STAT3 had been downregulated after the presurgical 7-day RO4929097 treatment phase (Supplemental Figure S4). Their reemergence at recurrence could represent a consequence of Notch inhibition, however, an evolution towards a more mesenchymal phenotype has also been reported in recurrent gliomas especially following radiation exposure (29, 30). The absence of recurrent controls, inter-patient molecular tumor heterogeneity and the limitation to two genes limits further conclusions.
Patient Outcomes
Although not a study objective, and limited by the small sample size, phase I design, and the fact that most patients had tumor recurrence prior to initiation of treatment, we performed an exploratory analysis of survival. After a median follow-up of survivors of 24 months, the Kaplan-Meier median OS for glioblastoma patients was 21 months (95% CI 12–28) and median PFS was 13 months (95% CI 7–13). At the time of analysis, all patients with AA (n=4) were alive (31+, 37+, 41+ and 53+ months). Two of them progressed at 31 and 46 months, respectively, while two other remained free of progression at 37+ and 41+ months.
Exploratory Tissue Correlates
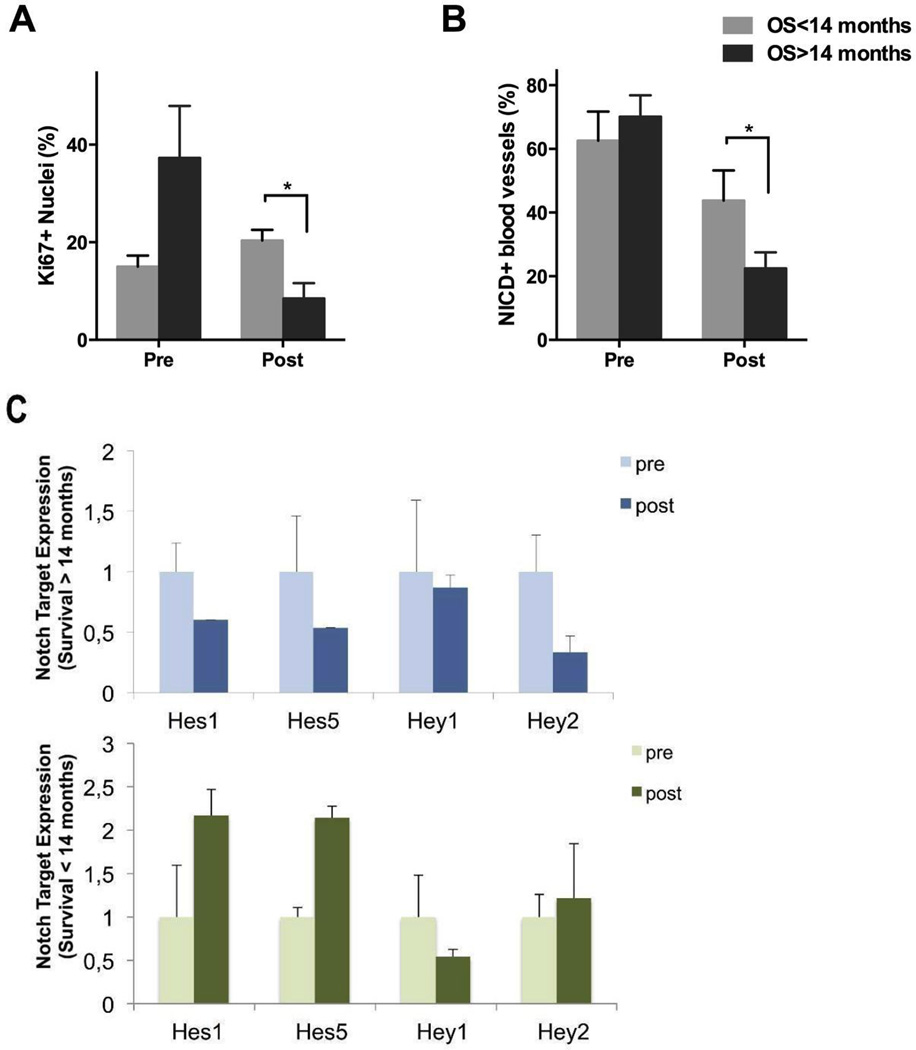
To determine the potential association of tissue characteristics and patient survival, we conducted correlative analyses in an exploratory manner. Patients on whom both pre- and post-treatment tissue was available for immunohistochemical and molecular studies were divided into two subgroups (“favorable” and “poor” survival) based on a cut-off value of 14 months, chosen based on median survival in glioblastoma historical controls. The patient subgroup demonstrating favorable survival exhibited a substantial decrease in Ki-67 from 37.3 ± 10.7 % at baseline to 8.5 ± 3.2 % after 7 days of RO4929097, while the patients in the poor survival group experienced an increase in proliferation from 15.0 ± 2.3 % to 20.4 ± 2.2 % (Figures 5A). Changes in NICD expression on vascular structures were also more significant in the group exhibiting better survival (22.5 ± 5.0 % vs. 43.8 ± 9.0%; Figure 5B). Interestingly, gene expression values of Notch targets were more consistently downregulated in the favorable survival subgroup compared to their poor survival counterparts (Figure 5C). To exclude the possibility that these differences are due to variations in drug levels, average tissue drug levels in both favorable and poor survival groups were determined, showing no statistical differences in tumor tissue levels (0.15 ± 0.03 µM vs. 0.19 ± 0.05 µM; p=0.36), and thus suggesting differential sensitivity to Notch inhibition among tumors. While the number of available specimens is very limited, these data suggest that early tissue analysis (e.g. after 7 days of treatment) could have a predictive value as to the ability to achieve target inhibition and a biological response.
Figure 5. Association of overall survival time with Ki-67 proliferation index, NICD+ vessel ratio, and Notch downstream target gene expression.
A and B, Ki-67 proliferation index and NICD+ blood vessel ratios grouped according to patient survival groups (n=7). C, Expression of downstream Notch target genes (Hes1, Hes5, Hey1 and Hey2) by qRT-PCR in patients with poor and good overall survival times (n=5). Values are expressed as mean ± S.E.M. (*p<0.05; OS = overall survival).
Discussion
In this study we report a phase I trial of oral gamma-secretase/Notch inhibitor RO4929097 in combination with standard treatment in newly diagnosed glioblastoma and WHO grade III AA. The trial design allowed for extensive exploratory studies, including neuro-imaging and laboratory experiments evaluating drug brain penetration, target modulation and potential mechanisms of resistance.
The combination of RO4929097, temozolomide and RT was feasible and well tolerated. Toxicity frequencies were similar to those observed in previous trials of RO4929097 and temozolomide (19). The 20 mg daily dose was deemed the RP2D. PK parameters of RO4929097 were in good agreement with data reported in phase I studies of RO4929097 given as a single agent or combined with cediranib (19, 23). Clinical investigations of the distribution of anticancer drugs into brain tumors are uncommon and it is uncertain how well preclinical models reflect drug uptake. To fully evaluate the potential of RO4929097 for the treatment of brain tumors, drug concentrations were determined in tissue resected from contrast enhancing regions of the tumor, representing tissue with a disrupted BBB, as well as non-enhancing regions in which the BBB is intact. While considerable variability among patients was observed, drug concentrations in the low micromolar range were achieved in both regions of the tumors. The observed intratumoral concentrations of the drug were comparable to its IC50 values against human brain cancer cell lines (13, 18). Interestingly, a differential expression profile of Notch ligands, receptors and downstream targets was initially observed between enhancing versus non-enhancing areas, strengthening the hypothesis that the drug preferentially penetrated areas with BBB breakdown where it then produced a more potent, inhibitory effect on Notch targets. However, prolonged drug exposure seemed to bypass the BBB concern, as samples obtained at later times displayed similar drug concentrations in enhancing and non-enhancing areas. Heterogeneity of the tumor and adequately characterizing the integrity of the BBB in the region from where the tumor was resected are underlying concerns for studies such as this, as discussed in a recent review (30).
Previous data showed that radiation and Notch inhibition may act synergistically in impairing tumor survival (11, 13). In parallel to the clinical trial, we conducted experiments on tumor explants, which further support a role for the combination of radiation, temozolomide and Notch inhibition. Analyses of glioblastoma organotypic explants showed a decrease in proliferation upon RO4929097 treatment (in comparison to immunohistochemical studies on parent tumor tissue), and an even more pronounced decrease in viability when exposed to triple therapy. Additionally, the proportion of CD133+ cells decreased after exposure to RO4929097, highlighting the role of the Notch pathway in maintenance of GSC. Armed with an appropriate sample size, tumor explants could be developed into a powerful screening platform with a predictive capacity for clinical response within a short time span, acting as a “functional biopsy”. Tumor explants play a particularly important role in assessing tumor response whereby modulation of environmental factors, such as endothelial cells, is relevant. In the case of Notch inhibition, we have previously shown that the in vitro response of cell lines is often different from and less predictive of true Notch activity, in comparison to 3D explants which maintain endothelial cell integrity (11).
Our attempts to identify reliable markers of drug activity included quantitative immunohistochemistry for NICD - the immediate product of gamma-secretase-mediated cleavage of the inner domain of the Notch receptor - as well as quantitative gene expression. The immunohistochemical data showed a significant decrease in NICD expression in matching specimens obtained before and after treatment. While intratumoral heterogeneity remains a concern, these data suggest that sufficient drug exposure and target modulation were achieved. In comparison, gene expression data were more challenging to interpret. Downregulation of Notch targets was inconsistent following one-week exposure to RO4929097, although samples obtained after prolonged treatment displayed a more pronounced modulatory effect. Notably, Hes1, a major gene target downstream of the Notch pathway remained essentially unchanged in some patients despite other evidence in support of pathway inhibition, such as the decrease in HES5 and in NICD. This raises the question on whether this could be a result of non-canonical, Notch receptor-independent activation in these patients, known to occur via a variety of signaling pathways such as hedgehog, FGF, Wnt, STAT or BMP signaling attributed to the Activin receptor-like kinase (Alk)1 activity (31, 32).
The gene expression in tumor tissue obtained at later time points, after completion of RT, suggests that such tumors recurred in the context of persistently low Notch activity. Compared to the original tumors, recurrences exhibited significant neo-angiogenesis as evidenced by a statistically significant increase in PECAM (i.e. CD31, a robust endothelial marker) - as well as in the expression of angiopoietins. Coupled with the decrease in Notch activity in vascular structures seen upon initiation of therapy, these data would suggest that angiogenesis in the recurrent tumor developed independently of Notch signaling. Notch inhibition has also been reported to affect the biology of abnormal glioblastoma tumor vessels (33–35). Indeed, our DCE MRI studies showed an early decrease in tumor blood perfusion upon RO4929097 treatment. This was associated with a reduction in tumor vessels expressing the cleaved form of the Notch receptor (NICD), as shown by immunohistochemistry. While prolonged treatment further reduced the number of NICD-positive blood vessels (achieving values similar to those of normal brain tissue), microvascular density was unchanged and gene expression data showed a marked increase in PECAM expression in these recurrent tumors. These data suggest that recurrent tumors switched to a Notch-independent angiogenic profile. Both the sustained activation of Hes1 and the neo-angiogenesis probably represent aspects of a resistance phenomenon that develops in the context of sustained Notch inhibition. These findings also support the concomitant use of anti-VEGF agents in future trials (28, 32, 36).
This current study is limited by the inherent difficulties of translational research in human subjects, especially in phase I settings. Tissue availability was not comprehensive, particularly for samples from the time of initial biopsy, usually obtained in community hospitals and yielding small amounts of paraffin fixed tissue from stereotactic procedures. Nonetheless, we attempted to optimize molecular analyses on all tissues in an effort to glean maximal information. Finally, while the data support the ability of GSIs to cross the BBB and modulate targets in the brain, tumor recurrence could not be avoided, suggesting a role for combination therapies. Drug levels could be further optimized, perhaps with the use of alternative agents with more favorable PK profiles as compared to RO4929097. In fact, for the time being, further development of RO4929097 has been halted, in a decision made by the manufacturer prior to results of this study becoming available. Several other GSIs are in development and may provide viable alternatives for further exploring this approach.
In summary, we describe a proof-of-concept study within the scope of a phase I clinical trial investigating the use of gamma-secretase/Notch inhibition in combination with temozolomide and RT in newly-diagnosed glioblastoma or anaplastic astrocytoma. This regimen was safe, well tolerated and appears to be a promising addition in the effort to control these tumors. Correlative studies demonstrate the ability of this drug to achieve sufficient brain penetration, although higher doses and continuous administration may be beneficial. Tissue studies also provided insights into the mechanisms of action and resistance associated with Notch inhibition, confirming its effects on GSC populations and angiogenesis in humans, and supporting a potential synergism with RT and temozolomide. The study also highlights the tumor’s ability to adapt to alterations in its microenvironment, thereby switching to a Notch-independent form of angiogenesis. These findings further endorse the necessity of targeting multiple signaling pathways concomitantly in gliomas, and highlight the biological differences in targeting otherwise normal developmental pathways, as opposed to targeting specific mutations. It is clear that Notch inhibition, alone or in combination with radiation, is insufficient to fully control tumor progression; but it does demonstrate promise as a targeted biological tool that counteracts tumor stem cell-like behavior by curbing self-renewal, and possibly angiogenesis. More effective anti-proliferative agents and other environmental modulators will still be necessary as adjuncts.
Supplementary Material
Statement of Translational Relevance.
In this Phase 0/I trial, gamma-secretase/ Notch inhibitor RO4929097 was added to chemoradiotherapy to treat glioblastomas and anaplastic astrocytomas. A comprehensive integrated translational approach demonstrated tumor penetration and effective target modulation of the drug, as measured by effects on neuro-imaging, gene expression, modulation of glioma stem cells, and promising efficacy.
Acknowledgments
RO4929097 was provided by Roche, through CTEP.
Funding
The study was partially funded by NIH/NCI through grants 3P30CA008748-47S2 (NCI Cancer Clinical Investigator Team Leadership Award, Omuro awardee) and U01CA069856. Additional funding was provided by B*Cured Foundation, Philadelphia Foundation and Have a Chance Foundation.
Abbreviations
- AA
anaplastic astrocytoma
- AUC
area under the curve
- BBB
blood brain barrier
- CIS
Cancer-Initiating Cells
- Cmax
peak concentration
- DCE
dynamic contrast-enhanced
- GSC
glioma stem cells
- GSI
gamma-secretase inhibitor
- Ktrans
volume transfer coefficient
- MTD
maximum tolerated dose
- NICD
Notch intracellular domain
- PK
pharmacokinetic
- RT
radiotherapy
- TMZ
temozolomide
- Vp
ratio of fractional plasma volume
- MSKCC
Memorial Sloan Kettering Cancer Center
Footnotes
The authors have declared that no conflict of interest exists.
Authors Contributions
Study Design: AO, VT, PI, NT; Conducting experiments: RX, SF, KKP, AO, VT; Patients enrollment and treatment: KB, PG, TK, LD, EP, CN, CG, TC, AH, VT, AO; Acquiring data: DB, RX, KKP, AO; Analyzing data: All authors; Manuscript writing: RX, VT and AO; Manuscript review: All authors.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 5.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 6.Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 7.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Abravanel DL, Belka GK, Pan TC, Pant DK, Collins MA, Sterner CJ, et al. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J Clin Invest. 2015;125:2484–2496. doi: 10.1172/JCI74883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 11.Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der Heijden M, Moayedpardazi H, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28:1019–1029. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito N, Fu J, Zheng S, Yao J, Wang S, Liu DD, et al. A high Notch pathway activation predicts response to gamma secretase inhibitors in proneural subtype of glioma tumor-initiating cells. Stem Cells. 2014;32:301–312. doi: 10.1002/stem.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dantas-Barbosa C, Bergthold G, Daudigeos-Dubus E, Blockus H, Boylan JF, Ferreira C, et al. Inhibition of the NOTCH pathway using gamma-secretase inhibitor RO4929097 has limited antitumor activity in established glial tumors. Anti-Cancer Drug. 2015;26:272–283. doi: 10.1097/CAD.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 16.Purow B. Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol. 2012;727:305–319. doi: 10.1007/978-1-4614-0899-4_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of Notch signaling. Onco Targets Ther. 2013;6:943–955. doi: 10.2147/OTT.S33766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luistro L, He W, Smith M, Packman K, Vilenchik M, Carvajal D, et al. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res. 2009;69:7672–7680. doi: 10.1158/0008-5472.CAN-09-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30:2348–2353. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LoConte NK, Razak AR, Ivy P, Tevaarwerk A, Leverence R, Kolesar J, et al. A multicenter phase 1 study of gamma -secretase inhibitor RO4929097 in combination with capecitabine in refractory solid tumors. Invest New Drug. 2015;33:169–176. doi: 10.1007/s10637-014-0166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz-Padilla I, Hirte H, Oza AM, Clarke BA, Cohen B, Reedjik M, et al. A phase Ib combination study of RO4929097, a gamma-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest New Drug. 2013;31:1182–1191. doi: 10.1007/s10637-013-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strosberg JR, Yeatman T, Weber J, Coppola D, Schell MJ, Han G, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer. 2012;48:997–1003. doi: 10.1016/j.ejca.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahebjam S, Bedard PL, Castonguay V, Chen Z, Reedijk M, Liu G, et al. A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503) Brit J Cancer. 2013;109:943–949. doi: 10.1038/bjc.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter S, Bedard PL, Chen EX, Clarke BA, Tran B, Hotte SJ, et al. A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575) Invest New Drug. 2014;32:243–249. doi: 10.1007/s10637-013-9965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- 26.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 27.Stockhausen MT, Kristoffersen K, Poulsen HS. The functional role of Notch signaling in human gliomas. Neuro-oncology. 2010;12:199–211. doi: 10.1093/neuonc/nop022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–6072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neuro-Oncol. 2011;104:629–638. doi: 10.1007/s11060-011-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pourquie O. Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell. 2011;145:650–663. doi: 10.1016/j.cell.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woltje K, Jabs M, Fischer A. Serum induces transcription of hey1 and hey2 genes by alk1 but not notch signaling in endothelial cells. PLOS One. 2015;10:e0120547. doi: 10.1371/journal.pone.0120547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 34.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 35.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 36.Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141:140–149. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.