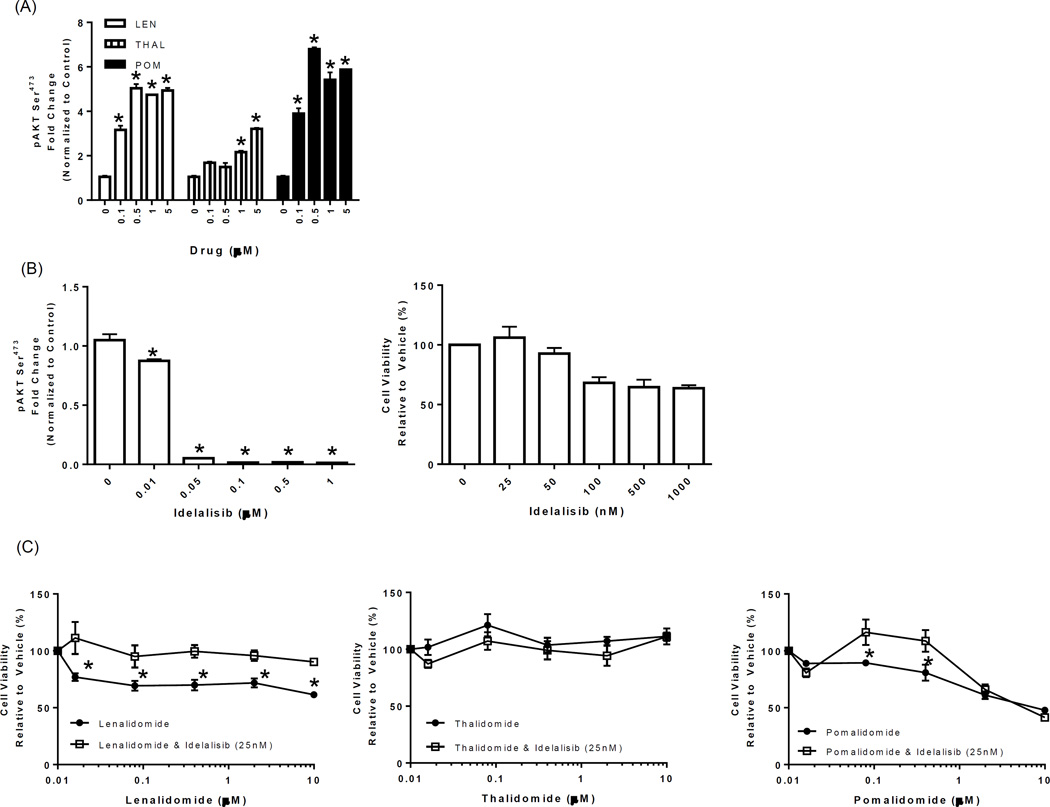
Figure 4. Idelalisib blocks LTP-mediated stimulation of PI3K at nanomolar concentrations and reverses LTP-mediated inhibition of proliferation.
(A) DAUDI cells were incubated with vehicle or LTP (0.1–5 µM) for 24 hours, and PI3K activity evaluated in cell lysates using a pAKTSer473 ELISA. pAKTSer473 values were normalized to those of the total AKT (also determined by ELISA), and the fold change was compared to that with the vehicle control. (B) Idelalisib (0.01–1 µM) was added to DAUDI cells for 24 hours, the suppression of PI3K was measured using a pAKTSer473 ELISA (left panel), and normalization was as described above. DAUDI cells were treated with either vehicle or increasing concentrations of idelalisib (25–1000 nM) for 72 hours, cell viability was determined using the WST-1 reagent, and results were expressed as the percentage viability relative to the vehicle control, which was arbitrarily set at 100% (right panel). “*” denotes p values of <0.05 compared to the vehicle control in A and B. (C) DAUDI cells were treated with LTP (0.016–10 µM) alone or in combination with idelalisib (25 nM) for 72 hours, cell viability was determined using the WST-1 reagent, and results were expressed as the percentage viability relative to the vehicle control, which was arbitrarily set at 100%. “*” denotes p values of <0.05 comparing the single-agent LTP to the idelalisib combination.