Abstract
The clinical efficacy of immune cytokines used for cancer therapy is hampered by elements of the immunosuppressive tumor microenvironment such as TGF-ß. Here we demonstrate that FIST15, a recombinant chimeric protein composed of the T cell stimulatory cytokine IL-15, the sushi domain of IL15Rα and a TGF-β ligand trap, can overcome immunosuppressive TGF-β to effectively stimulate the proliferation and activation of natural killer (NK) and CD8+ T cells with potent antitumor properties. FIST15-treated NK and CD8+ T cells produced more IFNγ and TNFα compared to treatment with IL-15 and a commercially available TGF-β receptor-Fc fusion protein (sTβRII) in the presence of TGF-β. Murine B16 melanoma cells which overproduce TGF-β were lysed by FIST15-treated NK cells in vitro at doses ~10-fold lower than NK cells treated with IL-15 and sTβRII. Melanoma cells transduced to express FIST15 failed to establish tumors in vivo in immunocompetent murine hosts and could only form tumors in beige mice lacking NK cells. Mice injected with the same cells were also protected from subsequent challenge by unmodified B16 melanoma cells. Lastly, mice with pre-established B16 melanoma tumors responded to FIST15 treatment more strongly compared to tumors treated with control cytokines. Taken together, our results offer a preclinical proof of concept for the use of FIST15 as a new class of biological therapeutics that can coordinately neutralize the effects of immunosuppressive TGF-β in the tumor microvironment while empowering tumor immunity.
Introduction
Extensive studies documenting the use and efficacy of cytokine-based immunotherapy for cancer in the pre-clinical setting have largely failed to materialize into significant improvements in clinical therapy for cancer patients. To date, interleukin 2 (IL2) remains the only FDA-approved cytokine monotherapy for the treatment of cancer (1). Cytokines, in particular those belonging to the common gamma-chain (γc) family, IL2, IL4, IL7, IL9, IL15, and IL21, have been the most studied for their potential anti-tumor properties (2). These cytokines typically signal via a heterodimeric receptor complex, sharing the use of a common gamma-chain, CD132, for ligand binding and signaling, in addition to a ligand-specific receptor alpha-chain (3). In the case of IL2 and IL15, the receptor complex is heterotrimeric, owing to the use of an additional IL2/15 receptor beta-chain (βc/CD122) (4). These γc cytokines are thought to mediate their anti-tumor effects through the activation of innate and adaptive arms of the immune system (2).
Indeed, IL2 has been shown to potently activate tumor infiltrating CD8+ T cells and natural killer (NK) cells, enhancing their ability to induce tumor cytolysis and clearance (5). However, its serious and potentially life-threatening toxicity profile combined with its low objective responsive rate has made it a last line treatment in patients with metastatic disease (6). In contrast to IL2, IL15 has less systemic toxicity and has been shown to be well tolerated in non-human primate models and preliminary human clinical trials (7, 8). Whereas IL2 can actually promote tumor growth by inducing regulatory CD4+ T cell (Tregs) formation and activation-induced cell death (AICD) of CD8+ T cells exposed to high concentrations of cognate antigen (9, 10), IL15 has no discernible effect on Treg formation and resists AICD by inducing expression of anti-apoptotic proteins (11). Moreover, IL15 has a non-redundant, but critical, role in the formation and maintenance of memory CD8+ T cells; an immunological effect particularly desired in cancer immunotherapy because it hints at durable, long-lasting protection against future tumor formation (12, 13).
Despite the beneficial effects of IL15 and other γc cytokines, we and others have demonstrated that tumor-derived immunosuppressive factors severely abrogate the efficacy of cytokine and cell-based immunotherapies (14, 15). Transforming growth factor-beta (TGF-β) is one such immunosuppressive factor overexpressed by the vast majority of solid tumors (16). TGF-β is a pleotropic cytokine involved in cell growth and differentiation, acting as a tumor suppressor early in tumorigenesis, but takes on oncogenic functions late in tumorigenesis, as the tumor becomes insensitive to its growth inhibitory effects. TGF-β secreted by tumors promotes angiogenesis, potentiates the ability of tumors to metastasize from its primary site, and inhibits the effector functions of tumor infiltrating lymphocytes (17). CD8+ T cells and NK cells are particularly sensitive to these inhibitory effects, being unable to efficiently proliferate, produce proinflammatory cytokines, and activate cytolytic pathways in the presence of TGF-β (18, 19). Beyond directly inhibiting these subsets to evade immune responses, TGF-β can convert and recruit immune cells to promote tumor growth, such as tumor associated macrophages, myeloid tumor derived suppressor cells (MDSCs), CD4+ Tregs, and tolerogenic dendritic cells (DCs) (20).
To enhance the efficacy of pro-inflammatory IL15 for use in cancer immunotherapy, we here describe a novel protein therapeutic consisting of IL15 and the sushi subunit of the IL15Rα chain fused to a TGF-β ligand trap. Termed FIST15 (Fusion of Interleukin 15 with IL15Rα-sushi and TGF-β receptor), we detail the design and use of this protein as a bifunctional biopharmaceutical for use in cancer immunotherapy. We found that the addition of the IL15Rα-sushi domain to IL15 in the FIST15 fusion protein enhances its biological activity and rescues its signaling activity compared to a fusion of IL15 to the TGF-β ligand trap without the IL15Rα-sushi domain (FIST15Δsushi). The use of two tandem TGF-β receptor ectodomains (type II; TβRII-ECD) recently described (21) and modified for use in FIST15, allows it to effectively neutralize TGF-β signaling. FIST15 treatment enhanced the capacity of NK and CD8+ T cells to produce critical anti-tumor cytokines, such as IFNγ and TNFα, as well as their expression of cytolytic effector molecules. In vitro, FIST15 was superior to control cytokines at stimulating NK cell mediated killing of B16-F0 melanoma and MC-38 colon adenocarcinoma cells. We found that FIST15 predominately acts through NK cells to mediate control and clearance of B16-F0 tumor in vivo, and that FIST15 treatment of immunocompetent mice bearing pre-established B16-F0 tumors resulted in significant delay in tumor outgrowth and improvement in overall survival.
Materials and Methods
Cell lines, in vitro culture, and FIST15 protein generation
cDNA containing mouse IL15/IL15Rα-sushi domain (Genscript) was subcloned 3′ to cDNA of tandem TGF-β receptor ectodomain. A 5′ VEGF signal peptide, Tobacco etch virus protease cleavage site, and 8X His-Tag was added to produce FIST15 cDNA. HEK293T cells were transfected and the supernatant collected after 48h, concentrated with Centricon Plus-70 (Millipore, Billerica, MA). Concentration of FIST15 and IL15 was quantified by IL15/IL15Rα complex ELISA (eBioscience). Protein expression was confirmed by immunoblotting of supernatants by anti-mouse IL15 and TGF-βRII antibodies (R&D). Retroviral particles containing FIST15 or GFP cDNA were generated with 293-GP2 packaging cells (Clontech) and used to infect B16-F0 cells to generate B16-FIST15 and B16-GFP cells. HEK293T and B16-F0 cells were obtained from American Type Cell Culture (ATCC), aliquoted, and used within 6 months of recovery from cryopreservation. MC-38 colon adenocarcinoma cell lines were a kind gift from Dr. Pnina Brodt (McGill University) (22) and were last authenticated by SNP array profiling in 2014. Cell lines were maintained in DMEM medium supplemented with 10% FBS and 50 U/ml penicillin and streptomycin (Wisent).
Immunoblotting and STAT5 phosphorylation by intracellular flow cytometry
Splenocytes from C57Bl/6J mice were used as responder cells in immunoblots and phosphorylated STAT5 intracellular flow cytometric staining. Splenocytes were maintained in RPMI1640 media (Corning) with L-glutamate, HEPES, β-mercaptoethanol, 50U/ml penicillin and streptomycin, and 10% FBS (Wisent). Cells were serum-starved for 2 hours prior to stimulation with FIST15, FIST15Δsushi or equimolar control cytokines (recombinant mouse IL15, TGFβRII-Fc chimera; sTβRII, and IL15+sTβRII, R&D) with or without recombinant mouse TGF-β1 (R&D). Immunoblot samples were lysed after 20 minutes of stimulation with indicated cytokines, separated by SDS-PAGE, and blotted for pSTAT3 (pY705) pSTAT5 (pY694), STAT3, STAT5. All antibodies were obtained from Cell Signaling Technologies. Intracellular flow cytometric analysis for pSTAT5, splenocytes were treated by FIST15 or cytokine controls for timepoints indicated before fixation with Lyse/Fix Buffer and permeabilization with Perm Buffer III (BD Biosciences), staining with CD3, CD8, and NK1.1, and an AF488-conjugated antibody for pSTAT5 (pY694) and analyzed using a FACSCanto II (BD Biosciences) and FlowJo software v9.6 (TreeStar Inc).
Cell phenotyping, cytokine profiling, and proliferation analysis
Splenocytes derived from C57Bl/6J mice were cultured in RPMI media (untreated), stimulated with FIST15, or IL15 and sTβRII for 72 hours at 37°C with TGF-β1. For cell surface marker staining, cells were resuspended in PBS with 2% FBS, incubated with anti-mouse FcR III/II for 15 minutes and labeled with conjugated antibodies specific for CD3, CD4, CD8, CD11b, CD19, CD25, CD27, CD44, CD45, CD49b, CD62L, CD95L, CD122, CD314, Granzyme B, KLRG1, H-2kb, IFNγ, IL2, NK1.1, TNFα, TRAIL. For cytokines and effector molecule expression, Leukocyte Activation Cocktail (2μl/ml, BD Biosciences) was used to stimulate cells for an additional 4-6 hours at 37°C before cell surface staining, fixation/permeabilization with BD Cytofix/Cytoperm (BD Biosciences). Analysis and presentation of distributions was done using FlowJo and Simplified Presentation of Incredibly Complex Evaluations (SPICE, NIH) v5.35. Proliferation was assessed utilizing carboxyfluorescein succinimidyl ester (CFSE) CellTrace (Life Technologies) pre-labeled lymphocytes stimulated with FIST15 or control cytokines for 72 hours with or without TGF-β1 and analyzed by flow cytometry. Replication indices were calculated using Prism v6.0 (GraphPad Software).
In vitro cytotoxicity assays
NK cells were isolated from splenocytes of C57Bl/6J mice with the EasySep Mouse NK Cell Isolation Kits (Stemcell Technologies). Population purity assessed by flow cytometry was >95%. B16-GFP cells were allowed to adhere overnight before purified splenic NK cells from C57Bl/6J mice were added at an Effector:Target ratio of 20:1. Doses of FIST15 or control cytokines (0.32pM-1000pM) were added to the co-culture for 48 hours. Floating cells and debris were washed away and adherent cells were trypsinized, washed, and analyzed for GFP positivity. %B16-GFP+ survival was calculated by dividing the number of GFP+ events in each condition by GFP+ events from B16-GFP cultures without NK cells. MC-38 cytotoxicity assay was conducted in a similar manner. MC-38 cells were pre-labeled with CFSE and %MC-38 survival was calculated on the basis of CFSE+ events. Event counts were normalized to AccuCheck counting beads (Thermo Fisher). Granzyme B and caspase 6 serine protease activity was assayed by PanToxiLux cytotoxicity assay kit (OncoImmunin). Co-cultures of NK cells and B16-F0 with FIST15 or controls were set up as described above. 24 hours post co-culture, fluorogenic substrate was added to the culture for 2 hours, after which cells were trypsinized, washed, and stained with CD45. Substrate cleavage was assayed by flow cytometry to determine (FL-1 fluorescence) on CD45×, B16-F0 cells.
Mice and in vivo experiments
Wildtype C57Bl/6J, B6.129S2-Cd4tm1Mak/J (CD4+ T cell deficient, Cd4−/−), B6.129S2-Cd8atm1Mak/J (CD8+ T cell deficient, Cd8−/−), B6.129S2-Ighmtm1Cgn/J (B cell deficient, μMT), and C57Bl/6J-Lystbg-J/J (NK cell defective, Beige) female mice (6 to 8 weeks old) were obtained from The Jackson Laboratory. In tumor implantation experiments, mice were implanted subcutaneously in the flank with 1 × 106 of the indicated cell type (B16-F0, B16-GFP, or B16-FIST15). In rechallenge experiments, 1 × 106 B16-F0 cells were implanted subcutaneously on the contralateral flank two weeks after B16-FIST15 challenge. In the therapeutic model experiments, 1 × 106 B16-F0 tumor cells were subcutaneously implanted into C57Bl/6J mice and allowed to develop palpable tumors before treatment was initiated with 4 intraperitoneal doses of FIST15 (~3ug/dose), equimolar cytokine controls, or PBS, every second day. Tumor volume was calculated with the formula: ((length×width2)/2) with unblinded measurements of tumor dimensions taken with an electronic caliper (Fisher Scientific).
Statistical analysis
Values are presented as mean ± SEM. Statistical significance was determined by Students t test (two-tailed), one-way ANOVA, in instances of multiple comparisons, followed by Tukey’s test, or log-rank test for survival experiments using Prism v6.0 (GraphPad Software). * P < 0.05, ** P < 0.01, *** P < 0.001, ****, P < 0.0001.
Results
Generation and characterization of murine IL15/IL15Rα/sTβRII fusion protein: FIST15
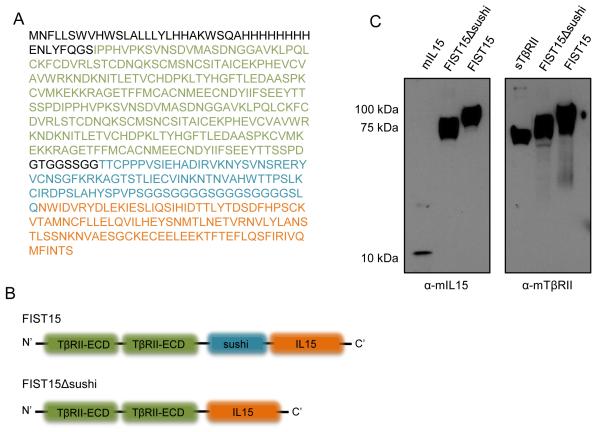
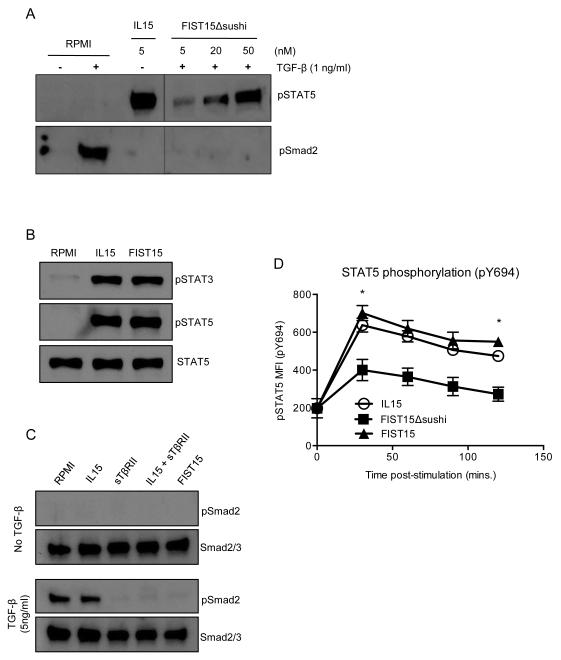
We generated a plasmid construct encoding the fusion of murine IL-15 and the sushi domain of the IL15 receptor-alpha (IL15Rα-sushi; Thr 34 to Pro 109) to the C’-terminus of two tandem TGF-β traps consisting of portions of the TGF-β receptor ectodomain (TβRII-ECD) containing the conserved, structured regions required for TGF-β binding (Gln 74 to Thr 180) flanked by amino acid linkers derived from the unstructured region of the TβRII-ECD. Cloned in frame N’-terminal to these three domains were a VEGF signal peptide to direct for protein secretion to the extracellular space, an 8x-His tag, and a Tobacco etch virus (TEV) protease cleavage site for downstream protein purification (Fig. 1A and B). The mature, secreted FIST15 protein is 506 amino aids in length and migrates as a ~100 kDa protein under reducing conditions on SDS-PAGE (Fig. 1C). A variant plasmid of this construct excluding the IL15Rα-sushi domain (FIST15Δsushi) was also generated, creating a protein of approximately 75 kDa under reducing conditions in size (Fig. 1C). Both constructs were able to inhibit TGF-β signaling by inhibiting Smad2 phosphorylation in unfractionated murine splenocytes treated with recombinant TGF-β1 (Fig. 2A and C). To analyze the effect of these constructs on the IL15 signaling pathway, STAT3 and STAT5 phosphorylation status of unfractionated splenocytes was interrogated after FIST15 and FIST15Δsushi treatment. STAT5 phosphorylation was significantly diminished with FIST15Δsushi treatment compared to IL15. Addition of the IL15Rα-sushi domain in FIST15 rescued STAT5 phosphorylation and induced STAT3 phosphorylation to levels comparable to equimolar IL15 treatment as observed by immunoblot (Fig. 2B). Intracellular flow cytometric analysis of CD8+ T cells also showed that addition of IL15Rα-sushi domain significantly enhanced pSTAT5 signaling compared to FIST15Δsushi to levels, bringing STAT5 activation to levels comparable to equimolar IL15 treatment (Fig. 2D).
Figure 1.
Design and expression of murine FIST15 and FIST15Δsushi. FIST15 peptide sequence is shown in (A) with a schematic of critical domains in FIST15 and FIST15Δsushi shown in (B). Immunoblot of murine IL15 and murine TGF-β receptor (type II), on conditioned supernatant of human embryonic kidney (HEK293T; transduced with SV40 Large-T antigen) cells transfected with a plasmid containing cDNA of FIST15 and FIST15Δsushi in (C). TβRII-ECD: sequences binding to TGF-β and linker amino acids derived from the unstructured regions of the TGF-β receptor ectodomain, sushi: interleukin-15 receptor alpha chain-sushi domain, IL15: interleukin-15.
Figure 2.
FIST15 and FIST15Δsushi signaling properties. Primary murine splenocytes were used as responder cells in immunoblots. FIST15Δsushi is capable of neutralizing TGF-β1 mediated phosphorylation of Smad2 (pSmad2; Ser465/467), but was deficient at inducing phosphorylation of STAT5 (pSTAT5; Tyr694) (A). With the addition of the IL15Rα-sushi domain to FIST15Δsushi (FIST15), STAT5 phosphorylation is rescued and STAT3 phosphorylation (pSTAT3; Tyr705) is found to be comparable to equimolar IL15 stimulation (B). Addition of the IL15Rα-sushi domain to FIST15Δsushi did not alter its ability to neutralize TGF-β1 (C). Flow cytometric analysis of pSTAT5 (Tyr694) on primary splenic CD8+ T cells upon IL15, FIST15Δsushi, and FIST15 treatment over 2 hours (D). Mean fluorescence intensity (MFI) of the pSTAT5 signal ± SEM on primary splenic CD8+ T cells was determined. Statistical significance was determined by Student t test comparing FIST15 to FIST15Δsushi. *, P < 0.05. No significant differences were detected between FIST15 and IL15 treatment at any time points.
FIST15 induces NK and CD8+ T cell proliferation and activation in TGF-β rich environments
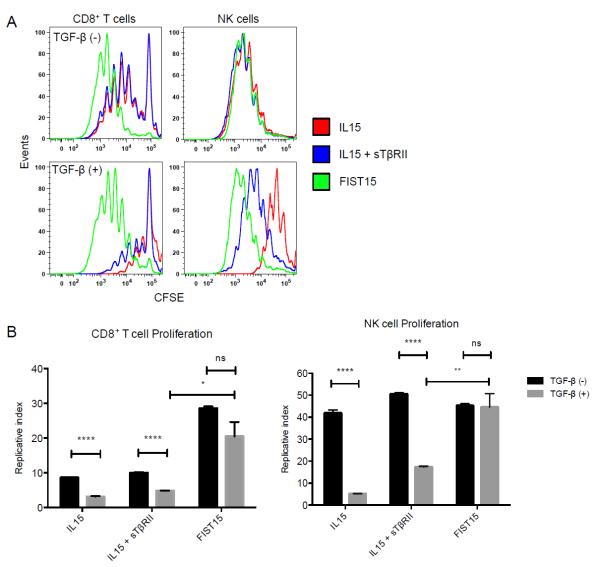
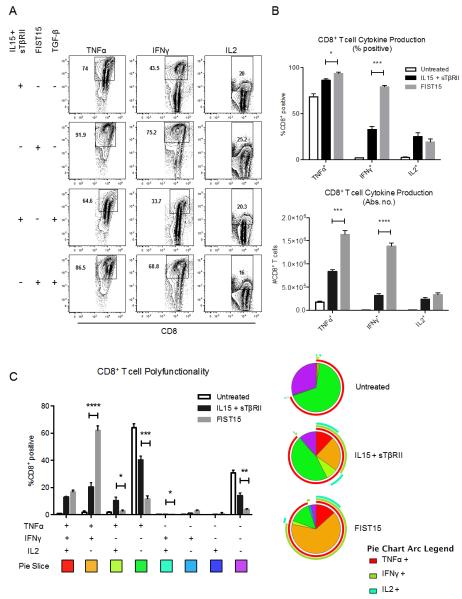
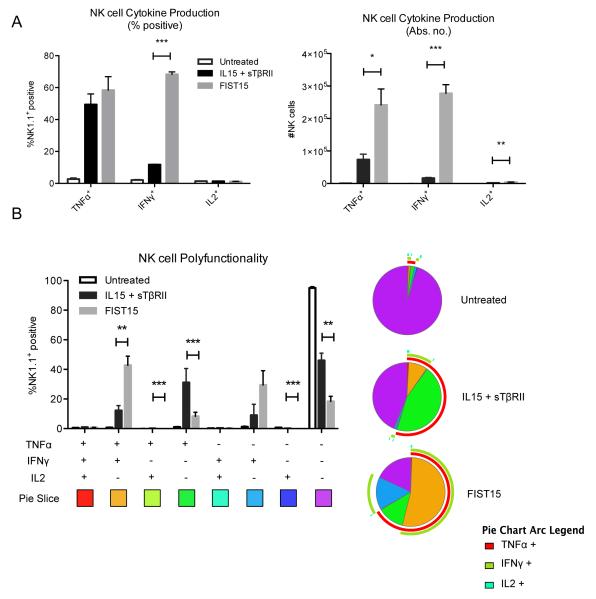
In order to determine the physiological effects of FIST15 treatment on lymphomyeloid cells, we cultured splenocytes with FIST15 or equimolar control cytokines (IL15+sTβRII) for 72 hours and assessed the proliferation and phenotype of major cellular subsets. An increased proportion of NK and CD8+ T cells were noted after three days of FIST15 culture when compared to splenocytes that were untreated (Supplementary Fig. S1A). CFSE-labeling of these subsets revealed that the proportional increases seen were due to FIST15 driven proliferation (Fig. 3A). FIST15 was superior to equimolar treatment with IL15 alone and IL15+sTβRII at inducing CD8+ T cell proliferation; while FIST15 treated NK cells proliferated at a comparable rate to control cytokine treatment (Fig. 3B). CD4+ T cells and B cells did not proliferate in response to FIST15 (Supplementary Fig. S1B). In the presence of TGF-β1, FIST15 significantly enhanced NK and CD8+ T cell proliferation compared to control treated cells (Fig. 3A and B). Upon PMA/ionomycin stimulation, FIST15 significantly augmented the ability of CD8+ T cells to produce TNFα and IFNγ, but not IL2, compared to control treated cells in the presence of exogenous TGF-β1 (Fig. 4A and B). FIST15 significantly enhanced the proportion of TNFα+IFNγ+ double positive CD8+ T cells compared to IL15+sTβRII treated CD8+ T cells (Fig. 4C). While IL15+sTβRII treatment enhanced the proportion of CD8+ T cells that were TNFα+IL2+ and IFNγ+IL2+, the relative contributions of these two subsets to the entire population are low (<15%). FIST15 treatment also significantly decreased the proportion of triple negative CD8+ T cells.
Figure 3.
FIST15 induces NK and CD8+ T cell proliferation in the presence of TGF-β. Murine splenocytes labeled with CFSE were cultured with IL15, IL15 + sTβRII, or FIST15 (1000 pM) in the presence or absence of TGF-β1 (5 ng/ml) for 72 hours before flow cytometric analysis in (A). The replicative index, representing fold-expansion of cells that undergo division ± SEM of CD8+ T cells and NK cells are shown in (B). Representative plots are shown in (A) and data in (B) are from three independent experiments. Statistical significance was determined by Student t tests. * P<0.05, ** P < 0.01, ****, P < 0.0001, P > 0.05 was considered to be not significant (ns).
Figure 4.
FIST15 treatment is superior at inducing CD8+ T cell production of TNFα and IFNγ, but not IL2, compared to IL15 and sTβRII treatment. Primary splenic CD8+ T cells treated with IL15 + sTβRII or FIST15 (500 pM) for 72 hours in the presence or absence of TGF-β1 (5 ng/ml), followed by a 6 hour stimulation with PMA/ionomycin/brefeldin A, were analyzed by flow cytometry for TNFα, IFNγ, and IL2 production. Gating was determined by isotype controls. Representative plots are shown in (A). Histograms comparing the mean percentage of RPMI (untreated), IL15 and sTβRII, and FIST15 (500 pM) treated CD8+ T cells for 72 hours in the presence of TGF-β1 (5 ng/ml) followed by a 6 hour PMA/ionomycin/brefeldin A stimulation expressing TNFα, IFNγ, or IL2 ± SEM (top panel) and the absolute number of cells expressing these cytokines (bottom panel) from three independent experiments are shown in (B). Representative pie charts displaying the proportion of CD8+ T cells from the above treatment conditions producing each combination of TNFα, IFNγ, and IL2 and histograms displaying the mean ± SEM for each combination are shown in (C). Data from three independent experiments are shown. Statistical significance was determined by Student t tests. * P < 0.05, ** P < 0.01, *** P < 0.001, ****, P < 0.0001.
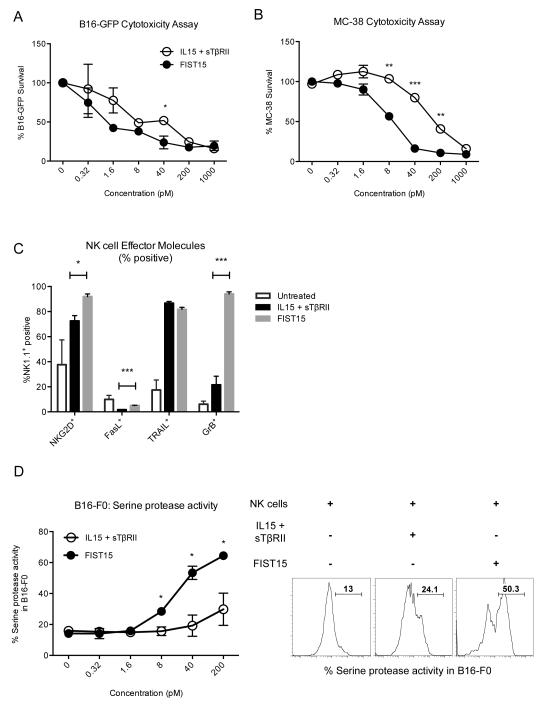
FIST15 enhances NK cell effector molecule expression and augments in vitro cytolysis of B16-F0 melanoma and MC-38 colon adenocarcinoma cells by NK cells
Similar to CD8+ T cells, FIST15 treated NK cells also displayed significantly increased production compared of IFNγ compared to control treated NK cells under TGF-β rich conditions, when stimulated with PMA/ionomycin (Fig. 5A). Absolute numbers of NK cells secreting IFNγ, TNFα, or IL2 were also significantly increased compared to control cytokine treatment (Fig. 5A). NK cells treated with FIST15 also exhibited enhanced cytokine polyfunctionality, by significantly increasing the proportion of TNFα+IFNγ+ double positive NK cells, and decreasing the proportion of NK cells that fail to produce any cytokine upon stimulation (Fig. 5B). To test whether FIST15 stimulated NK cells could inhibit tumor growth in vitro, we utilized the murine B16-F0 melanoma cell line transduced to express GFP (B16-GFP) in a cytotoxicity assay. B16-GFP cells were allowed to adhere overnight before being placed in co-culture with murine splenic NK cells for 48 hours at increasing concentrations of FIST15. Adherent B16-GFP cells were then trypsinized and analyzed by flow cytometry for GFP+ events. While FIST15 had no direct effect on B16-GFP growth (data not shown), NK cells in the presence of FIST15 significantly diminished B16-GFP growth (Fig. 6A). Using non-linear regression, a concentration of FIST15 or IL15 + sTβRII required to inhibit 50% of B16-GFP growth (IC50) could be determined. Compared to treatment with equimolar IL15 and sTβRII treated NK cells, FIST15 achieved an IC50 approximately 10.5-fold lower (2.0 pM, FIST15 vs 21.0 pM, IL15 + sTβRII). Low or lack of MHC-I expression on target tumor cells, such as in B16-F0 melanoma has been known to spontaneously induce NK cell-mediated cytolysis (Supplementary Fig. S2). In order to test whether FIST15 could stimulate NK cells to lyse MHC-I expressing cells, we utilized syngeneic MC-38 colon adenocarcinoma cells (Supplementary Fig. S2). MC-38 cells labeled with CFSE were allowed to adhere overnight before co-culture with NK cells and FIST15 or control cytokines. MC-38 cells were also susceptible to NK cell-mediated lysing in the presence of FIST15, despite their MHC-I expression (Fig. 6B). However, increased concentrations of FIST15 were required to induce comparable lysis to B16-F0 cells. FIST15 was more potent compared to IL15+sTβRII at inducing MC-38 cytolysis, achieving an IC50 approximately 14.7-fold lower (10.5 pM, FIST15 vs 155.1 pM, IL15 + sTβRII). We next investigated the effect of FIST15 treatment on the expression of effector molecules associated with NK cell cytotoxicity. We found significantly higher surface expression of death receptor ligands, such as Fas ligand, and the NK cell activating receptor, NKG2D, on the surface of FIST15 treated NK cells, compared to controls (Fig.6C). Intracellularly, FIST15 treated NK cells produced significantly higher amounts of granzyme B, a serine protease released from cytotoxic granules, which activate caspases in target cells to initiate apoptosis. To determine if this was the mechanism by which FIST15 treated NK cells induced B16-F0 cell death; we utilized a fluorochrome-based cytotoxicity assay to measure the activity of granzyme B and caspase 6 in B16-F0 cells co-cultured with NK cells and FIST15 or control cytokines (Fig. 6D). After 24 hours of co-culture, higher serine protease activity was found within B16-F0 cells cultured with NK cells and FIST15 compared to controls.
Figure 5.
FIST15 augments NK cell cytokine production. Primary splenic NK cells cultured in RPMI media (untreated), treated with IL15 + sTβRII, or FIST15 (500 pM) for 72 hours in the presence of TGF-β1 (5 ng/ml), followed by a 6 hour stimulation with PMA/ionomycin/brefeldin A, were analyzed by flow cytometry for TNFα, IFNγ, and IL2 production. Histograms present the mean of the percentage of NK cells ± SEM (left panel) and absolute number of NK cells ± SEM (right panel) expressing each cytokine (A). Representative pie charts displaying the proportion of NK cells from the above treatment conditions producing each combination of TNFα, IFNγ, and IL2 and histograms displaying the mean ± SEM for each combination are shown in (B). Data from three independent experiments are shown. Statistical significance was determined by Student t tests * P < 0.05, ** P < 0.01, *** P < 0.001.
Figure 6.
FIST15 enhances NK cell cytotoxicity and cytolytic effector molecule expression. B16-GFP cells were co-cultured with purified splenic NK cells in the presence of IL15 and sTβRII or FIST15 (0.32 – 1000 pM) for 48 hours. %B16-GFP survival was calculated by dividing the number of GFP+ events in each condition by the number of GFP+ events in control wells containing B16-GFP cells without NK cells. Event counts were normalized to counting beads. Each point represents the mean percent survival ± SEM (A). CFSE-labeled MC-38 cell survival in the presence of NK cells with IL15 and sTβRII or FIST15 was similarly measured. %MC-38 survival was calculated by dividing the number of CFSE+ events in each condition by the number of CFSE+ events in control wells containing CFSE-labeled MC-38 cells without NK cells (B). The percentage of NK cells expressing NK activating receptor, NKG2D, Fas ligand (FasL), TNF-related apoptosis inducing ligand (TRAIL), and granzyme B (GrB) ± SEM after 72 hour treatment with FIST15 or control cytokines, followed by a 6 hour stimulation with PMA/ionomycin/brefeldin A, is shown in (C). Serine protease (granzyme B and upstream caspase) activity using a fluorogenic substrate was measured by flow cytometry in B16-F0 target cells following 24 hour NK cell co-culture with IL15 and sTβRII or FIST15 treatment (0.32 – 200 pM). Percent positivity (% serine protease activity) ± SEM is shown in (D). Inset shows representative plots for co-cultures at the 40pM dose. Gating was determined using B16-F0 cells cultured in the presence of the fluorogenic substrate in the absence of NK cells as a negative control. Data from two (A, B, and D) and three (C) independent experiments are shown. Statistical significance was determined by Student t tests * P < 0.05, ** P < 0.01, *** P < 0.001.
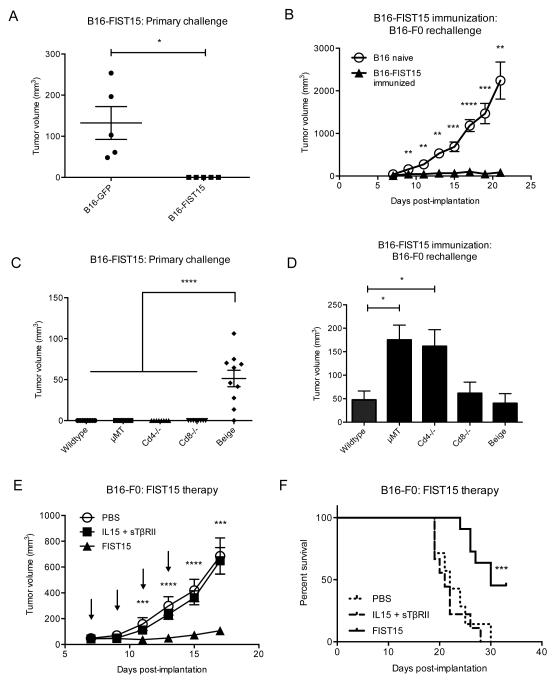
FIST15 antitumor effect in immunodeficient mice
In order to test the anti-tumor effects of FIST15 expression in vivo, we first sought to determine the effects of locoregional FIST15 expression in the tumor microenvironment. To do this, we stably transduced B16-F0 cells to produce FIST15. These cells, B16-FIST15, displayed similar in vitro growth kinetics to mock GFP-transduced B16-F0 cells (B16-GFP), but failed to form tumor in vivo in immunocompetent C57Bl/6J mice (Fig. 7A). Mice receiving B16-FIST15 tumor cells were also protected against subsequent rechallenge by wildtype B16-F0 melanoma cells (Fig. 7B). Mechanistically, we determined the immune subsets that mediated anti-tumor effect of FIST15 through the use genetic knockout mouse models lacking individual lymphomyeloid subsets. Consistent with our in vitro studies, we found that a lack of functional NK cells allowed for the establishment of tumors by B16-FIST15 cells (Fig. 7C). In contrast, lack of CD4+, CD8+ T cells, or B cells did not affect the ability of mice to mount anti-tumor responses against B16-FIST15 cells. We rechallenged these genetic knockout strains that had received B16-FIST15 cells with wildtype B16-F0 tumor and found that a lack of B cells and CD4+ T cells significantly correlated with increases in tumor volume (Fig. 7D).
Figure 7.
FIST15 inhibits B16-F0 tumor growth in vivo through NK cell activity and significantly delays growth of pre-established tumors. 1 × 106 B16-F0 transduced with FIST15 (B16-FIST15) or a vector containing GFP (B16-GFP) cells were implanted subcutaneously into the flank of immunocompetent C57Bl/6 mice (n=5, each) and monitored for tumor growth. Graph of tumor volumes at day 7 post-implantation ± SEM is shown in (A). Mice that had received B16-FIST15 (B16-FIST15 immunized, n=5) were rechallenged on day 14 post-implantation subcutaneously with 1 × 106 B16-F0 cells on the contralateral flank compared to naïve mice (B16 naïve; n=5) and monitored for tumor growth. Graph of tumor volumes from ± SEM is shown in (B). Syngeneic mouse strains lacking CD4+ (Cd4−/−), CD8+ (Cd8−/−) T cells, B cells (μMT), or functional NK cells (Beige) were implanted with 1 × 106 B16-FIST15 cells and tumor volume ± SEM from two independent experiments was measured on day 7 post-implantation in (C). Genetic knockout strains receiving B16-FIST15 were rechallenged 14 days post-implantation with 1 × 106 B16-F0 cells. Graph showing tumor volume ± SEM from two independent experiments at day 12 post-rechallenge is shown in (D). In the FIST15 therapeutic model, wildtype C57Bl/6J mice were implanted with 1 × 106 B16-F0 cells subcutaneously. Day 7 post-implantation, once palpable tumor had formed, mice were randomized into treatment groups receiving: PBS (n=7), IL15 + sTβRII (n=9), and FIST15 (n=11). Mice were given intraperitoneal injections every second day for 1 week (4 doses total, indicated by arrows) and monitored for tumor growth. Graph measuring tumor volume ± SEM is shown in (E). Survival of mice in the three groups depicted in a Kaplan-Meier plot is shown in (F). Representative data are shown from two (A, B, E, and F) independent experiments. Statistical significance was determined by Student t tests (A, B, and E), one-way ANOVA with Tukey multiple comparisons test in (C and D), and the log-rank test in (F). Statistical significance between IL15 + sTβRII and FIST15 conditions are denoted in (E and F). * P < 0.05, ** P < 0.01, *** P < 0.001, ****, P < 0.0001.
FIST15 treatment significantly inhibits growth of pre-established tumors
To test the efficacy of FIST15 as a therapeutic agent in the setting of pre-established tumor, we implanted 1 × 106 wildtype B16-F0 cells subcutaneously into the flank of immunocompetent C57Bl/6 mice and waited seven days for tumor to establish. We then treated tumor-bearing mice with intraperitoneal administration of FIST15, IL15 and sTβRII, or PBS every second day for 1 week (4 doses total) and monitored the mice for tumor progression and survival. FIST15 treated mice displayed a significant delay in tumor growth compared to PBS and IL15+sTβRII treated mice (Fig. 7E). We also observed a significant improvement in overall survival of FIST15 treated mice compared to controls (Fig. 7F).
Discussion
IL15 based monotherapy been met with moderate success in the pre-clinical arena utilizing experimental tumor models, with some groups reporting no effect, some effect, or significant effects on tumor growth (23, 24). Consistent with other groups, we have shown that modification of the IL15 domain of FIST15 with the addition of an IL15Rα-sushi domain increases its biological activity and is one of the potential reasons for the observed gain-of-function that FIST15 has over combinatorial IL15 and sTβRII treatment (25). IL15 is physiologically expressed in a complex with IL15Rα, which acts as a chaperone for IL15 (26). The complex is typically expressed on the surface of DCs, monocytes, and macrophages. This IL15/IL15Rα complex is then trans presented to cells expressing the γc (CD132) and the IL2/15Rβc (CD122), resulting in phosphorylation and activation of JAK1/STAT3 and JAK3/STAT5 pathways in the cells bearing these receptors (27). The addition of the IL15Rα-sushi domain in the FIST15 protein bypasses the requirement for IL15Rα-expressing cells to bind IL15 and transpresent to βc/γc-expressing cells. Mortier et al. have shown that complexing IL15 to soluble IL15Rα increases its affinity for βc/γc complexes over monomeric IL15, which could provide a mechanism by which FIST15 appears to have a strong gain-of-function (25). While this may be the case, we found no biochemical evidence that this increased affinity resulted in enhanced FIST15 mediated STAT5 signaling downstream by immunoblot (Fig. 2B), or more long-term, by intracellular flow cytometry of pSTAT5, when compared to IL15 treatment (Fig. 2D). Another potential reason for the gain-of-function observed in FIST15, particularly in our in vivo experimental systems, is increased stability and half-life of the molecule compared to monomeric IL15 and sTβRII molecules (28). Stoklasek et al. reported that human IL15/IL15Rα complexes increase the in vivo half-life of IL15 from 1 hour to over 20 hours in mice (29). Bioavailability of IL15 was also increased when complexed to IL15Rα, where maximum serum concentrations were elevated and peaked later than IL15 administration alone. FIST15 is similar in size to IL15/IL15Rα complexes and may also enhance its bioavailability and half-life over IL15 and sTβRII molecules (30).
FIST15 also seems to exhibit greater TGF-β neutralization than sTβRII on an equimolar basis (Fig. 3B). While the tandem TβRII-ECD TGF-β trap and sTβRII have been shown to neutralize TGF-β1 to similar degrees (21), sTβRII molecules consist of two TβRII-ECD domains linked by mouse IgG2a in a chimeric fashion. In our in vitro and in vivo systems where Fc-receptor expressing cells are present, sTβRII may be internalized and rendered ineffective, compared to FIST15, which contains no such domains.
In line with other studies, we have also observed that addition of TGF-β to primary cultures of NK and CD8+ T cells significantly inhibited their proliferation, activation, and effector functions. However, FIST15 treatment of these cells was able to overcome these deficits. Compared to IL15+sTβRII, FIST15 was superior at inducing IFNγ and TNFα production in CD8+ T cells, two cytokines that are critical in mediating anti-tumor responses (Fig. 5B). FIST15 also enhanced the proportion of TNFα+IFNγ+ cells. Such polyfunctionality has been correlated to the ability of CD8+ T cells to mount robust anti-tumor responses (31). The majority of FIST15 expanded CD8+ T cells retain markers of central memory phenotype T cells (CD62L+CD44+), but compared to IL15+sTβRII treated CD8+ T cells, express higher surface levels of CD25 (Supplementary Fig. S3A-D) and intracellular granzyme B (Supplementary Fig. S5), without appreciable differences in IL2 production, suggest that FIST15 might preferentially expand memory phenotype CD8+CD25hi T cells with a predilection for effector differentiation (31). The observation that FIST15 also significantly upregulates CD122/βc expression on CD8+ T cells compared to IL15+sTβRII treatment (Supplementary Fig. S3E) also fits with this hypothesis as CD25+CD122+ CD8+ T cells are generally marked for effector differentiation, whereas bonafide central memory CD8+ T cells are generally CD25−CD122+ (32). TGF-β has been known to suppress formation of memory CD8+ T cells, and tumor derived TGF-β has been proposed to do the same in vivo, resulting in inefficient priming of anti-tumor T cell responses and subpar memory recall responses (33, 34). In our in vitro model system, we were able to demonstrate that FIST15 can expand central memory phenotype CD8+ T cells, although these cells may be destined for further differentiation to effector cells.
Like CD8+ T cells, ex vivo stimulation of NK cells with FIST15 resulted in significantly higher expression of pro-inflammatory IFNγ, but not increased TNFα or IL2, compared to IL15+sTβRII treatment. However, the absolute number of NK cells expressing these cytokines post-FIST15 treatment became significant, owing to FIST15’s enhanced mitogenic response on NK cells in the presence of TGF-β1 (Fig. 5A). To test the effect of TGF-β on NK cell cytotoxicity, we specifically chose B16-F0 and MC-38 tumor cells, known to overexpress TGF-β or are insensitive to TGF-β growth-inhibitory effects, respectively, as targets in our in vitro killing assays. B16-F0 cells were more susceptible to lysis than MC-38 by NK cells, perhaps owing to their lack of MHC-I expression (Supplementary Fig. S2). Lack of inhibitory killer-immunoglobulin like receptors (KIRs) ligand expression, such as MHC-I on target cells, lowers the threshold for NK mediated killing (35). The ability of FIST15 treated NK cells to lyse target tumor cells is further correlated with their expression of cytotoxic effector molecules. With FIST15 treatment, significantly higher levels of granzyme B was detected within NK cells, resulting in enhanced serine protease activity in target tumor cells (Fig. 6C and D). While increased recruitment of CD8+ T cells into the tumor microenvironment has been well correlated to improved prognosis, the presence of tumor infiltrating NK cells and its effect on prognosis is more controversial. Recent studies have shown that tumor-infiltrating CD11b+CD27+ NK cells may convert into immunosuppressive myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment (36). We found, however, that FIST15 treated NK cells were predominately CD11blo with variable levels of CD27 expression (Supplementary Fig. S4A and B). Interestingly, we also observed that FIST15 treated CD8+ T cells also expressed higher levels of granzyme B, among other effector molecules associated with cytotoxicity, compared to control treated cells (Supplementary Fig. S5).
The inability of B16-F0 tumors expressing FIST15 to establish in immunocompetent mice could have pointed to FIST15 activation of either innate or adaptive immune cell subsets. To mechanistically ascertain the cellular subsets responsible for FIST15 mediated tumor rejection, we utilized genetic knockout animals. Beige mice, lacking functional NK cells, were the only mice where B16-FIST15 cells were able to form tumor (Fig. 7C). This would suggest that NK cells play a critical role in preventing tumor establishment in response to FIST15 secretion locoregionally. This is unsurprising, given most reports suggesting that IL15 mainly acts in vivo on NK cells to prevent tumor outgrowth (37). We were, however, surprised to see that B16-FIST15 tumors failed to grow in mice lacking CD8+ T cells, given the in vitro effects we had observed. We surmise that FIST15 may activate innate cells in vivo, in particular NK cells, to effectively clear tumor before the need for an adaptive response was required. However, FIST15 may have acted as an adjuvant for the adaptive arm of the immune system as B16-FIST15 implanted mice were protected against subsequent B16-F0 tumor rechallenge in wildtype mice (Fig. 7B and D).
When genetic knockout strains implanted with B16-FIST15 were rechallenged with B16-F0 tumors, we observed that μMT mice lacking B cells and CD4+ T cell-deficient mice uniformly developed tumors, and that these tumors grew at the fastest rate (Fig. 7D). This observation is in line with other studies suggesting an important role for antibody-mediated rejection of tumors, most likely via antibody-dependent cell-mediated cytotoxicity (ADCC) (38). Without the formation of tumor-specific antibodies, ADCC would be unable to occur, therefore resulting in enhanced tumor growth. Although NK cells are known to be the primary mediators of ADCC in vivo, it was interesting that B16-FIST15 immunized Beige mice were protected from B16-F0 rechallenge. Opsonization of tumor cells by antibodies may also play a role in B16-F0 clearance by macrophages and monocytes in an Fcγ receptor-dependent manner, but we were unable to test this hypothesis within our experimental model (38). We speculate that in our experimental model, CD4+ T cells likely provide help to B cells in promoting antibody formation against B16-F0 tumors as opposed to playing a direct cytolytic role.
In the therapeutic setting, the lack of any anti-tumor effect with IL15+sTβRII treatment compared to mice treated with PBS alone was an intriguing observation. As previously discussed, pre-clinical studies indicate that administration of recombinant IL15 alone seems to have limited impact on models of pre-established tumors (23). Administration of IL15 with soluble IL15Rα protein, or modification of IL15 with the addition of an IL15Rα domain in the form of a fusion protein, such as in FIST15, would likely enhance the molecule’s anti-tumor effects (29, 39). Similarly, strategies involving ligand traps or antibodies to sequester TGF-β have primarily been shown to be efficacious if primary tumors have been transduced to express such proteins, or in metatstatic models in which tumor cells have been systemically introduced (40). Differences in the physiologic clearance of both monomeric IL15+sTβRII may result in a lack of their biological effect from playing out in an additive manner to prevent tumor outgrowth. In contrast, FIST15 by virtue of being a single molecule would presumably act in the same and time space allowing for a more coordinated anti-tumor response.
To our knowledge, this is the first report of immunotherapy combining IL15 with TGF-β blockade. While other groups have shown that the use of IL15 can elicit anti-tumor effects, especially when it is complexed with IL15Rα, many of these studies failed to address the active mechanisms of immunoediting and immunosuppression within the tumor microenvironment, such as the secretion of TGF-β (39, 41, 42). In order fully realize the potential of IL15-based immunotherapy; combinatorial strategies with other immune and tumor modulating agents will likely be employed. Indeed, reports combining IL15 with chemotherapy, radiation, and other adjuvants have shown improved efficacy over the use of IL15 alone (7). Combinatorial therapy of IL15 with checkpoint blockade inhibitors, such as α-PDL1 and α-CTLA4, has shown particular promise, due in part to the effect of these agents on tumor-associated CD4+ Tregs, which potently suppress anti-tumor effects of NK and CD8+ T cells (20, 43). Similarly, FIST15 may act to inhibit tumor growth through sequestration of TGF-β and inhibition of CD4+ Treg formation. In a more recent study, an IL15 superagonist was found to be efficacious against tumors infiltrated with exhausted CD8+ T cells only when co-administered with PD-1 blockade (44). The benefit of FIST15’s ability to neutralize TGF-β, which has been implicated in CD8+ T cell exhaustion (34), may improve therapeutic efficacy against such tumors.
In conclusion, this study provides evidence that FIST15 can combine IL15 agonism to TGF-β neutralization into a single immunotherapeutic agent. FIST15 activates both innate and adaptive arms of the immune system, augmenting NK and CD8+ T cell functions, even in TGF-β rich conditions, such as that found within the tumor microenvironment. More importantly, FIST15 can inhibit tumor growth in vitro and in vivo, by enhancing NK cell activity. Given the dearth of pharmacological agents available for NK cell expansion, we believe that FIST15 holds great promise as a potential biologic to expand NK cells ex vivo for adoptive cell therapy or as a standalone immunotherapeutic agent for use in cancer, driving pro-inflammatory IL15 signaling on immune cells while attenuating an important axis of tumor immune evasion.
Supplementary Material
Acknowledgements
The authors thank Dr. Pnina Brodt for reagents, and all the members of the Galipeau research group for support. This work was supported by NIH R01AI093881.
Footnotes
Conflicts of interest: None declared.
References
- 1.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. Jama. 1994;271:907–13. [PubMed] [Google Scholar]
- 2.Pulliam SR, Uzhachenko RV, Adunyah SE, Shanker A. Common gamma chain cytokines in combinatorial immune strategies against cancer. Immunology letters. 2016;169:61–72. doi: 10.1016/j.imlet.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard WJ. Cytokines and immunodeficiency diseases. Nature reviews Immunology. 2001;1:200–8. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 4.Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4940–4. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel JP, Puri RK. Interleukin-2 toxicity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991;9:694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer immunology research. 2015;3:219–27. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–26. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. European journal of immunology. 2004;34:2480–8. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 10.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–61. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 11.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11445–50. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol. 2010;184:35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunological reviews. 2006;211:214–24. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penafuerte C, Galipeau J. TGF beta secreted by B16 melanoma antagonizes cancer gene immunotherapy bystander effect. Cancer immunology, immunotherapy : CII. 2008;57:1197–206. doi: 10.1007/s00262-008-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Yu Z, Muranski P, Palmer DC, Restifo NP, Rosenberg SA, et al. Inhibition of TGF-beta signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene therapy. 2013;20:575–80. doi: 10.1038/gt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Visser KE, Kast WM. Effects of TGF-beta on the immune system: implications for cancer immunotherapy. Leukemia. 1999;13:1188–99. doi: 10.1038/sj.leu.2401477. [DOI] [PubMed] [Google Scholar]
- 18.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U. Regulation of NK cell functions by TGF-beta 1. J Immunol. 1995;155:1066–73. [PubMed] [Google Scholar]
- 20.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwaagstra JC, Sulea T, Baardsnes J, Lenferink AE, Collins C, Cantin C, et al. Engineering and therapeutic application of single-chain bivalent TGF-beta family traps. Molecular cancer therapeutics. 2012;11:1477–87. doi: 10.1158/1535-7163.MCT-12-0060. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Wang N, Brodt P. Metastatic cells can escape the proapoptotic effects of TNF-alpha through increased autocrine IL-6/STAT3 signaling. Cancer research. 2012;72:865–75. doi: 10.1158/0008-5472.CAN-11-1357. [DOI] [PubMed] [Google Scholar]
- 23.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends in pharmacological sciences. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilipow K, Roberto A, Roederer M, Waldmann TA, Mavilio D, Lugli E. IL15 and T-cell Stemness in T-cell-Based Cancer Immunotherapy. Cancer research. 2015;75:5187–93. doi: 10.1158/0008-5472.CAN-15-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 × IL-15R alpha fusion proteins. The Journal of biological chemistry. 2006;281:1612–9. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 26.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 27.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunology letters. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. The Journal of biological chemistry. 2008;283:4189–99. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 29.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–80. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chertova E, Bergamaschi C, Chertov O, Sowder R, Bear J, Roser JD, et al. Characterization and favorable in vivo properties of heterodimeric soluble IL-15.IL-15Ralpha cytokine compared to IL-15 monomer. The Journal of biological chemistry. 2013;288:18093–103. doi: 10.1074/jbc.M113.461756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai N, Ikeda H, Tawara I, Shiku H. Tumor progression inhibits the induction of multifunctionality in adoptively transferred tumor-specific CD8+ T cells. European journal of immunology. 2009;39:241–53. doi: 10.1002/eji.200838824. [DOI] [PubMed] [Google Scholar]
- 32.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature reviews Immunology. 2012;12:180–90. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 33.Takai S, Schlom J, Tucker J, Tsang KY, Greiner JW. Inhibition of TGF-beta1 signaling promotes central memory T cell differentiation. J Immunol. 2013;191:2299–307. doi: 10.4049/jimmunol.1300472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell death & disease. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunology and cell biology. 2011;89:216–24. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 36.Park YJ, Song B, Kim YS, Kim EK, Lee JM, Lee GE, et al. Tumor microenvironmental conversion of natural killer cells into myeloid-derived suppressor cells. Cancer research. 2013;73:5669–81. doi: 10.1158/0008-5472.CAN-13-0545. [DOI] [PubMed] [Google Scholar]
- 37.Gillgrass AE, Chew MV, Krneta T, Ashkar AA. Overexpression of IL-15 promotes tumor destruction via NK1.1+ cells in a spontaneous breast cancer model. BMC cancer. 2015;15:293. doi: 10.1186/s12885-015-1264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:652–6. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bessard A, Sole V, Bouchaud G, Quemener A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Molecular cancer therapeutics. 2009;8:2736–45. doi: 10.1158/1535-7163.MCT-09-0275. [DOI] [PubMed] [Google Scholar]
- 40.Sheen YY, Kim MJ, Park SA, Park SY, Nam JS. Targeting the Transforming Growth Factor-beta Signaling in Cancer Therapy. Biomolecules & therapeutics. 2013;21:323–31. doi: 10.4062/biomolther.2013.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng L, Du X, Wang Z, Ju J, Jia M, Huang Q, et al. Hyper-IL-15 suppresses metastatic and autochthonous liver cancer by promoting tumour-specific CD8+ T cell responses. Journal of hepatology. 2014;61:1297–303. doi: 10.1016/j.jhep.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer immunology research. 2016;4:49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:6019–28. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desbois M, Le Vu P, Coutzac C, Marcheteau E, Beal C, Terme M, et al. IL-15 Trans-Signaling with the Superagonist RLI Promotes Effector/Memory CD8+ T Cell Responses and Enhances Antitumor Activity of PD-1 Antagonists. J Immunol. 2016 doi: 10.4049/jimmunol.1600019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.