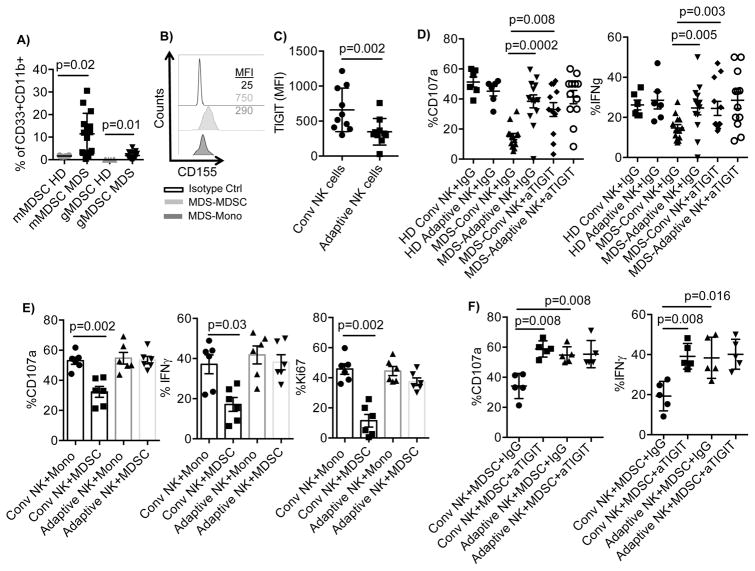
Figure 7. TIGIT-dependent suppression of conventional NK cells by MDS MDSCs.
A) PBMC (n=15) from MDS patients and healthy donors (n=6) were rested overnight, stained and the MDSC frequency were determined by flow cytometry. Monocytic MDSCs (mMDSCs) were defined as CD45+Lin−CD11b+CD33+HLA-DR−/lowCD14+ and granulocytic MDSCs (gMDSCs) as CD45+Lin−CD11b+CD33+CD15+. B) MDS-PBMC were stained for CD155 and gated for mMDSC and monocytes. Representative histograms are shown of 15. C) PBMCs (n=10) from MDS patients were rested overnight and evaluated for TIGIT expression by flow cytometry. D) PBMCs from healthy donors (HD, n=6) or MDS patients (n=13) were stimulated with IL-15 (10 ng/ml) in the presence of IgG control or anti-TIGIT, and anti-CD16 (1 ug/ml) for 6 hours and assessed for NK cell degranulation and IFN-γ production. E) Purified NK cells (n=6) from healthy blood donors were co-cultured with autologous monocytes or allogeneic MDSCs enriched from the blood of MDS patients at a 2:1 ratio in the presence of IL-15 (10 ng/ml) for 5 days. Following 6 hours stimulation with anti-CD16, degranulation and IFN-γ production was evaluated in conventional and adaptive NK cells by flow cytometry. F) Purified NK cells (n=5) from healthy blood donors were co-cultured with allogeneic MDSCs enriched from the blood of MDS patients at a 2:1 ratio in the presence of IL-15 (10 ng/ml), IgG control (10 ug/ml) and in the presence or absence of anti-TIGIT (10 ug/ml) for 5 days. 6 hours prior staining, cells were stimulated with anti-CD16 and degranulation and IFN-γ production was evaluated in conventional and adaptive NK cells by flow cytometry. Representative data are shown as mean ± SD, and statistical analysis were done on pooled data using the Student’s t test for (A), (C), (D), and Mann-Whitney test for (E) and (F).