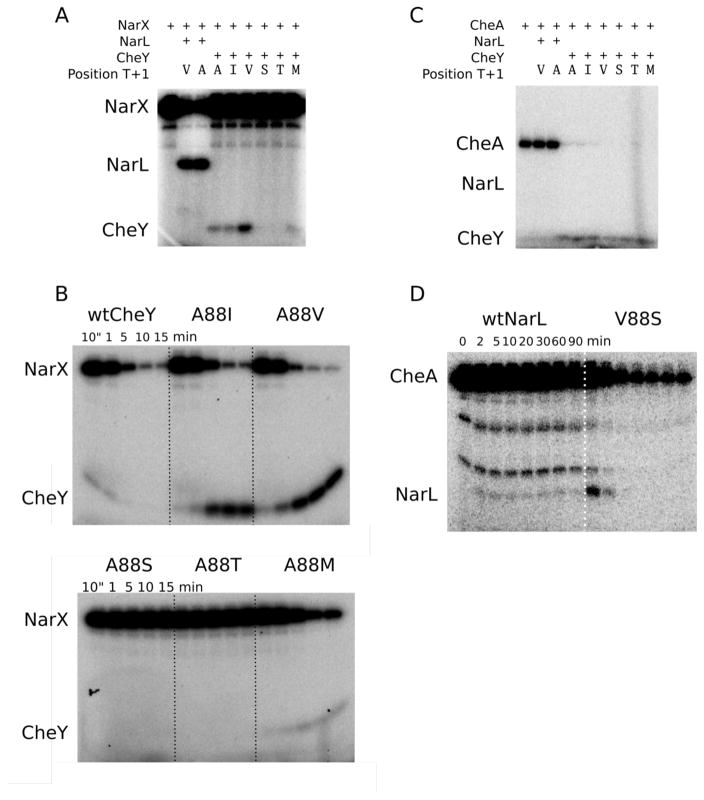
Figure 6.
Phosphotransfer permissiveness of the NarX/NarL and CheA/CheY two-component systems. (A) Ten second single time point partner and non-partner phosphotransfer from MBP-NarX227 to wild type or mutant NarL and CheY. The reactions used 1.5 μM MBP-NarX227 and 6 μM response regulator. (B) Time course experiments (10 sec to 15 min) for non-partner MBP-NarX227 to CheY phosphotransfer. The loss of [32P]MBP-NarX227-P, an indication of the MBP-NarX227 to CheY phosphotransfer rate, is similar (only four-fold difference in rate between CheYA88M and CheYA88V) for wild type and the three CheY variants with non-polar residues at position T+1. The variation in accumulation of [32P]CheY-P for these proteins largely reflects differences in autodephosphorylation rate constants (see Table 2). Neither CheYA88S nor CheYA88T accept phosphoryl groups from MBP-NarX227. (C) Single timepoint experiment (10 sec) for partner and non-partner phosphotransfer from CheA to wild type CheY, wild type NarL and corresponding position T+1 mutants. CheA can transfer a phosphoryl group to wild type CheY and each of the CheY mutants, but not to wild type NarL or NarLV88A. (D) In a longer time course experiment (2 min to 90 min), only non-specific phosphorylation was observed for wild type NarL by CheA, while NarLV88S was able to accept phosphoryl groups from CheA. Although phosphorylated NarLV88S only weakly accumulated in the first two time points, transfer from CheA and subsequent dephosphorylation by NarLV88S can explain the decrease in CheA-P.