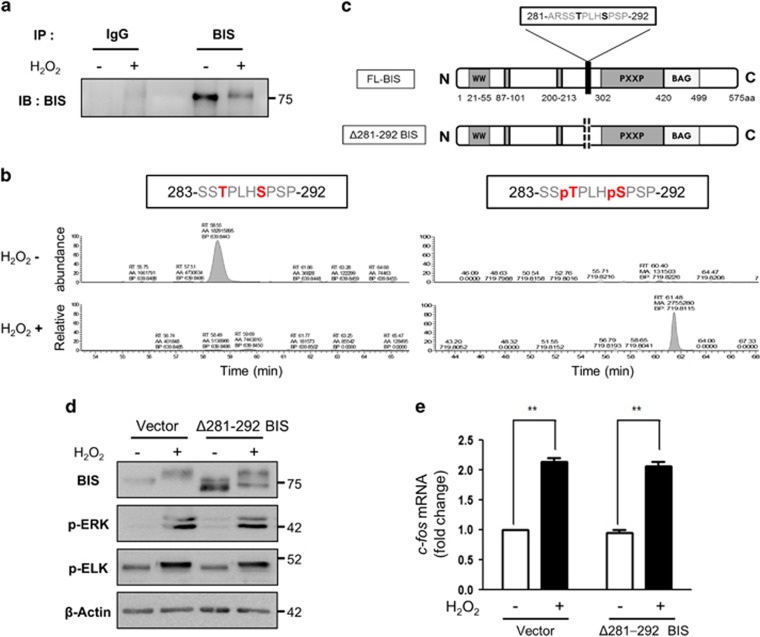
Figure 3.
Identification of BIS residues phosphorylated under oxidative stress. (a, b) Immunoprecipitation coupled with liquid chromatography/tandem mass spectrometry (LC-MS/MS) was used to identify BIS phosphorylation sites following H2O2 treatment. A172 cells were treated with 100 μM H2O2 for 3 h, and the BIS protein was immunoprecipitated from cell lysates with an anti-BIS antibody. (a) An aliquot of the immunoprecipitated proteins was analyzed by western blotting using an anti-BIS antibody. (b) The band corresponding to BIS in the immunoprecipitated complex was subjected to trypsin digestion and analyzed by LC-MS/MS; data were compared to computational predictions. The abundance of the unphosphorylated peptide 283-SSTPLHSPSP-292 and the phosphopeptide 283-SSpTPLHpSPSP-292 was quantified by peak area integration using the extracted ion chromatograms. (c) Schematic representation of FL-BIS and mutant BIS (with deletion of residues Ala281 to Pro292; Δ281–292 BIS). (d) A172 cells were transfected with the pCAGGS vector (Vector) or Δ281–292 BIS and treated 48 h later with 100 μM H2O2 for 3 h. Cell lysates were subjected to western blotting to assess the levels of BIS, p-ERK, and p-ELK proteins, with β-actin used as a loading control. (e) Relative levels of c-fos mRNA were evaluated by quantitative real-time PCR. Values represent the mean±s.e. of triplicate experiments. *P<0.05, **P<0.01. BIS, B-cell lymphoma (BCL)-2-interacting cell death suppressor; ERK, extracellular signaling-regulated kinase; ELK, ETS domain-containing protein; FL, full length.