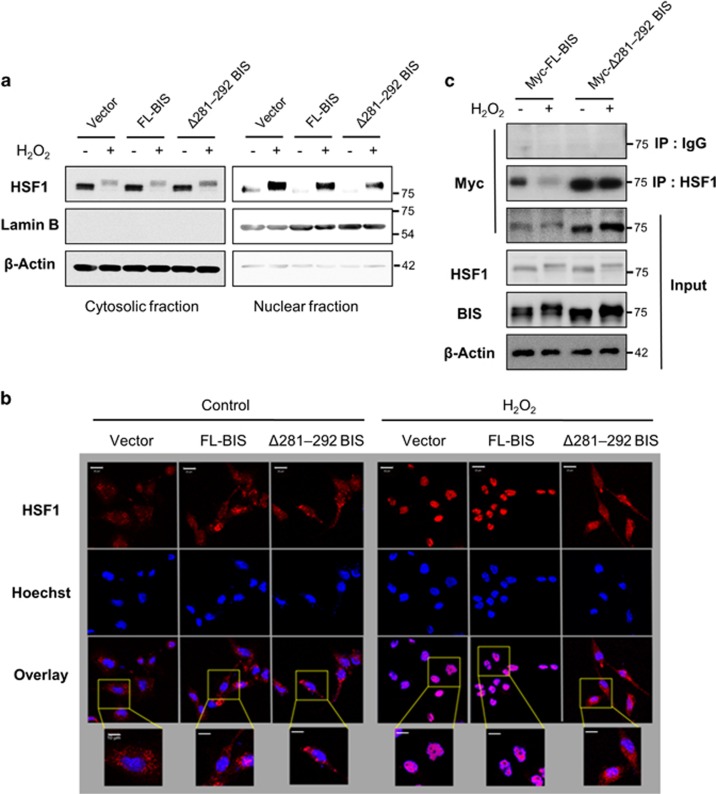
Figure 5.
Effect of the BIS mutation (Δ281–292) on HSF1 nuclear translocation. A172 cells were treated with 100 μM H2O2 for 3 h at 48 h after transfection of the indicated constructs. (a) Cytoplasmic and nuclear fractions were prepared, and BIS and HSF1 levels were detected by western blotting, with β-Actin and Lamin B used as loading controls. (b) The subcellular localization of HSF1 was visualized by confocal microscopy. For immunolabeling, cells were incubated with a primary antibody against HSF1 followed by a Texas Red-conjugated secondary antibody. Nuclei were stained with Hoechst. Bar=20 μm. Higher magnifications of the selected areas from overlay images are also provided. Bar=10 μm. (c) The interaction of BIS and HSF1 was verified by co-immunoprecipitation analysis after transfection of Myc-tagged-FL-BIS, or Myc-tagged-Δ281–292 BIS. Following exposure to H2O2, the cell lysates were immunoprecipitated with normal rabbit immunoglobulin G or anti-HSF1 antibody, followed by immunoblotting with an anti-Myc antibody. Total protein lysates were also subjected to western blot analyses with the indicated antibodies. BIS, B-cell lymphoma (BCL)-2-interacting cell death suppressor; HSF1, heat shock transcription factor 1.