Figure 8.
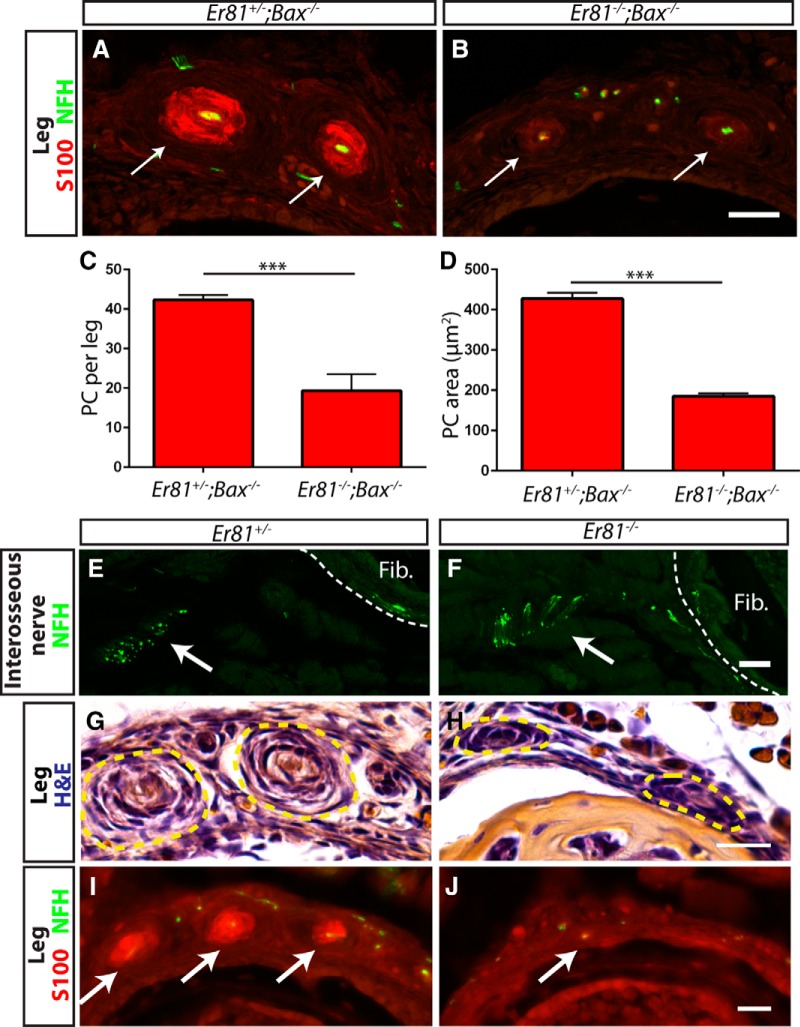
The primary deficit in Pacinian corpuscle formation in Er81 mutants is deficient axon/Schwann cell communication. A, B, Anti-S100 (red) and anti-NFH (green) staining of P3 Er81+/−;Bax−/− control (A) and Er81−/−;Bax−/− double-mutant (B) hindlimb sections. Arrows indicate Pacinian corpuscles. Note the lower expression level of S100 in double-mutant Pacinian corpuscles relative to controls. C, Quantification of S100+ Pacinian corpuscles in serial leg sections reveals a significant decrease in the number of corpuscles formed in double mutants relative to controls (42.33 ± 1.20 PC per leg in Er81+/−;Bax−/− controls, 19.33 ± 4.18 PC per leg in Er81−/−;Bax−/− double mutants; p = 0.006). D, Quantification of average cross-sectional area of Pacinian corpuscle inner cores reveals a significant decrease in the size of the remaining Pacinian corpuscles in double mutants (427.3 ± 14.7 μm2 in Er81+/−;Bax−/− controls, 185.3 ± 6.7 μm2 in Er81−/−;Bax−/− double mutants; p = 0.0001). E, F, Anti-NFH staining of P2 interosseous nerve shows intact innervation of fibula/interosseous membrane in both Er81+/− control (E) and Er81−/− mutant (F) mice. G, H, H&E staining of P2 hindlimbs shows early Pacinian corpuscles forming in the Er81+/− control (G). However, Schwann cells form much smaller, rudimentary Pacinian corpuscles in the Er81−/− mutant (H). I, J, Anti-S100 (red) and anti-NFH (green) staining shows that mature Schwann cells are present in corpuscles by P2 in the control (I) but are absent in the mutant (J). N = 3 animals, 6 legs per genotype. Scale bar, 20 μm. ***p < 0.001. Error bars indicate SEM.