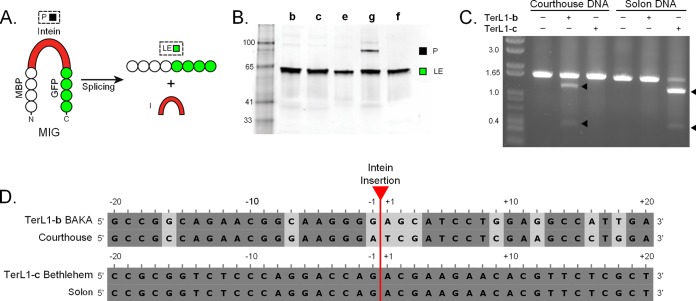
FIG 3 .
TerL inteins are splicing competent and can have active endonucleases. (A) MIG reporter system. The intein of interest was cloned between maltose binding protein (MBP) and GFP. Precursor (P) and ligated exteins (LE) are visualized by in-gel fluorescence. (B) TerL inteins are able to splice. Five representative TerL inteins cloned into the MIG reporter were assayed for splicing. Inteins are indicated by the insertion site letter. All five inteins investigated spliced quickly, primarily resulting in LE. Representative inteins are as follows: TerL1-b, BAKA gp6; TerL1-c, Bethlehem gp10; TerL1-e, Gaia gp2; TerL6-f, Chandler gp6; Pham3880-g, ScottMcG gp245. The numbers indicate the marker size in kDa. (C) TerL inteins have endonuclease activity. BAKA TerL1-b and Bethlehem TerL1-c inteins were tested for endonuclease activity against an inteinless TerL sequence from related phages, Courthouse and Solon, respectively. Cleavage products (black arrowheads) for both are ~1.3 kb and 0.4 kb. The DNA substrate was mixed with buffer, lysate with overexpressed unrelated TerL intein, or lysate with overexpressed related TerL intein. The numbers indicate the marker size in kb. (D) Sequence identity at TerL intein insertion sites. Sequence flanking the TerL intein insertion site (20 nucleotides up- and downstream) for each phage pair was analyzed, with high sequence identity among pairs (BAKA-Courthouse, 75%; Bethlehem-Solon, 100%). Conservation is shown by shades of gray. Data are representative of at least three independent experiments.