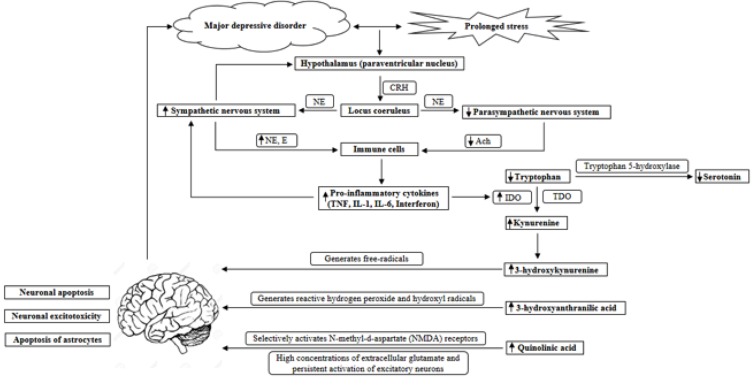
Fig. (1).
The interaction between stress, the autonomic nervous system, the immune system and the kynurenine pathway in the etiology of depression. The hypothalamus secretes CRH in response to stress, and from the paraventricular nucleus of the hypothalamus, CRH-containing neurons have projections to the locus coeruleus. The locus coeruleus sends direct projections to the sympathetic and parasympathetic preganglionic neurons, increasing sympathetic activity and decreasing parasympathetic activity through the activation of adrenoceptors. In turn, the activation of the sympathetic nervous system stimulates the release of CRH. When stress is prolonged, as in major depressive disorder, the sympathetic nervous system continues to be activated, with a lack of parasympathetic counter activity. As a result, NE and E levels are increased and ACh levels are decreased, which lead to an increased release of pro-inflammatory cytokines from immune cells. Pro-inflammatory cytokines, such as TNF, IL-1, IL-6 and interferons can induce IDO activity, which increases the KYN/tryptophan ratio. As a result, downstream metabolites, such as 3-hydroxykynurenine, 3-hydroxyanthranilic acid and quinolinic acid are increased, which all have neurotoxic effects on the brain. 3-hydroxykynurenine generates free-radicals and causes neuronal apoptosis. 3-hydroxyanthranilic acid generates highly reactive hydrogen peroxide and hydroxyl radicals. Quinolinic acid selectively activates N-methyl-d-aspartate (NMDA) receptors, and high concentrations of extracellular glutamate and persistent activation of excitatory neurons cause excitotoxicity. Therefore, the accumulation of quinolinic acid can result in neuronal excitotoxicity and the selective apoptosis of astrocytes. This can ultimately lead to neurodegenerative changes, which may render the brain susceptible to depression.