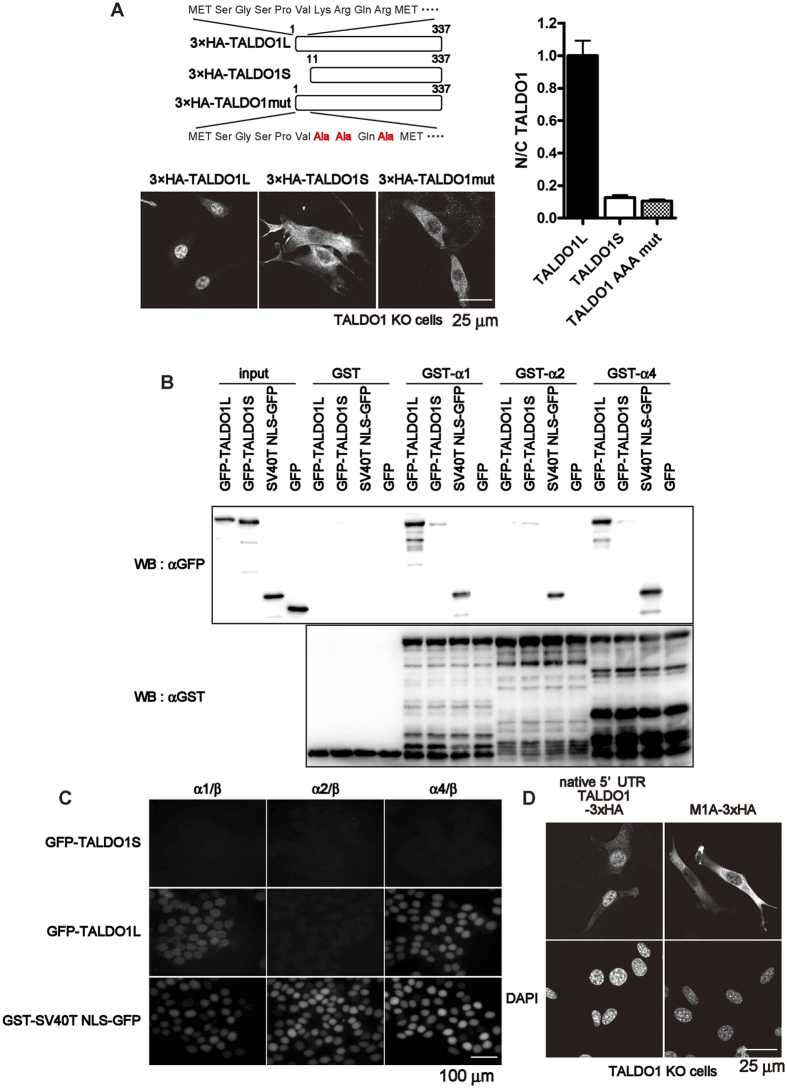
Figure 2. The 10 N-terminus amino acids are important for the nuclear localisation of TALDO1.
(A) Immunofluorescence images of TALDO1 isoforms. TALDO1 KO cells were transiently transfected with vectors expressing N-terminally HA-tagged TALDO1 long isoform (TALDO1L, 1–337 amino acids), TALDO1 short isoform (TALDO1S, 11–337 amino acids), or TALDO1 K7A, R8A, and R10A mutant (TALDO1 AAA mut) and stained with anti-HA antibodies. Quantification of the nuclear-cytoplasmic (N/C) ratio of TALDO1 isoforms and mutants. The ratio was normalised to that of TALDO1L (mean ± S.E.M., n = 23 cells). (B) Importin α subtypes recognize TALDO1L but not TALDO1S. GST pull-down assays were performed using GST, GST-importin α1 (GST-α1), GST-importin α2 (GST-α2), or GST-importin α4 (GST-α4) with TALDO1-GFP isoforms or GFP (as the negative control). The bound proteins were analysed by western blotting with an anti-GFP antibody. Used GST-fusion proteins (1 μg) were analysed by Coomassie Brilliant Blue staining. Asterisks indicate the full-length GST-fusion proteins. (C) In vitro transport assay revealed that GFP-TALDO1L protein, but not GFP-TALDO1S protein, was transported into the nucleus in the presence of importin α, importin β, Ran-GDP, and an ATP regenerating system. GST-SV40T NLS-GFP was used as a positive control. (D) Immunofluorescence images of TALDO1 KO cells expressing either native 5′ UTR-TALDO1-3×HA or M1A-3×HA. The transfected cells were stained as described above.