Figure 1. Schematic diagram showing thiopurine drug metabolism and genes implicated in thiopurine-induced toxicity.
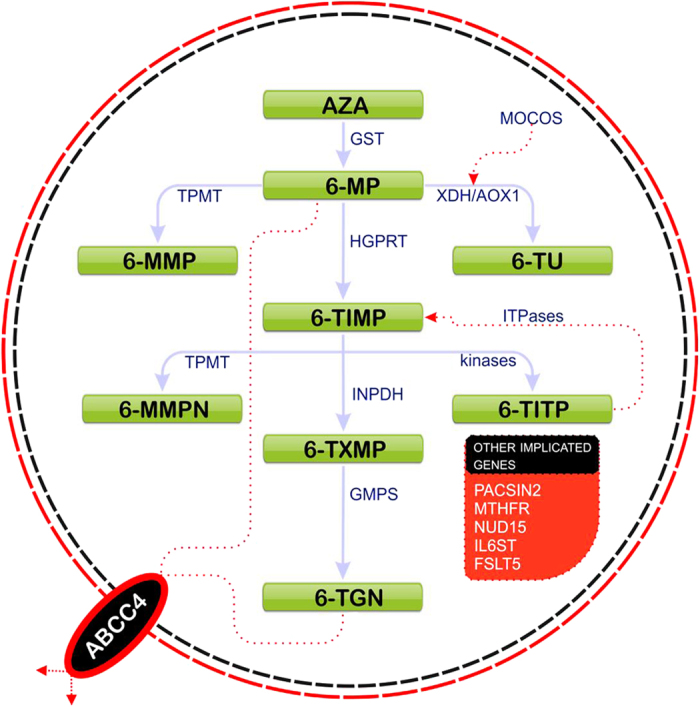
There are three main catabolic pathways for thiopurine drugs, following conversion of AZA to 6-MP: (1) Phosphorylation to 6-thioguanines (6-TGN) which are active metabolites; 6-MP is first converted by hypoxanthine guanine phosphoribosyl-transferase (HGPRT) to 6-thioinosine monophosphate (6-TIMP), which is then phophorylated to 6-TGN with inosine 5-monophosphate dehydrogenase (IMPDH1 and IMPDH2) and guanosine monophosphate synthetase (GMPS) (2) Methylation by of 6-MP by TPMT to form 6-methyl-MP (6-MMP) which is an inactive metabolite and not a substrate for IMPDH) (3) Catabolism of 6-MP to 6-thiouracil (6-TU) via xanthine dehydrogenase (XDH, synonym- Xanthine oxidase) or aldehyde oxidase 1 (AOX1). TPMT competes with IMPDH for their common substrate 6-TIMP to form 6- methylmercaptopurine nucleotides (6-MMPN). 6-TIMP can be phosphorylated by kinases to 6-thioinosine triphosphate (6-TITP), which can get dephosphorylated by inosine triphosphatase (ITPase) to form 6-TIMP again. Although the precise mode of action of thiopurines is still unclear, the most important mechanism is thought to be the incorporation of 6-TGNs into the cell DNA, resulting in an impaired DNA synthesis and cell death2,6. Abbreviations: ABCC4- ATP-binding cassette, sub-family C (CFTR/MRP), member 4; AZA- azathioprine; FSLT5- Follistatin-Like 5; GST- glutathione s-transferase; HGPRT- hypoxanthine phosphoribosyltransferase;IL6ST- Interleukin 6 signal transducer; 6-MP- 6- mercaptopurine; 6-MMPN- 6- methyl mercaptopurine nucleotides; MOCOS- Molybdenum cofactor sulfurase; MTHFR- Methyl-enetetrahydrofolate reductase ;NUDT15- Nudix (nucleoside diphosphate linked moiety X)-type motif 15; PACSIN2- Protein kinase C and casein kinase substrate in neurons 2; 6-TXMP- 6-thioxanthosine monophosphate)
