Fig. 1.
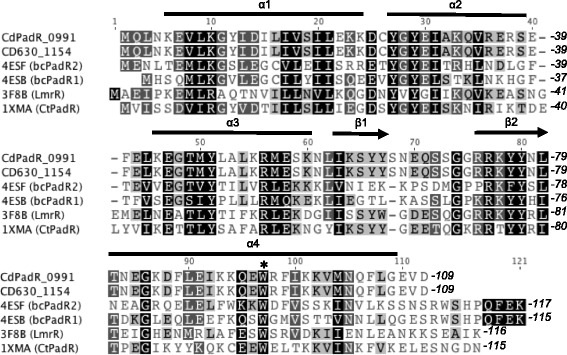
Amino acid sequence alignment of cdPadR1 from Clostridium difficile R20291 (CDR20291_0991) and 630 (CD630_1154) with structural homologues listed by accession number as follows: 4ESB (bcPadR2) from Bacillus cereus ATCC 10987 [10], 4ESF (bcPadR1) from B. cereus ATCC 14579 [10], 3F8B (LmrR) from Lactococcus lactis MG1363 [11], and 1XMA (CtPadR) from Clostridium thermocellum (unpublished). cdPadR1 and CD630_1154 share 100 % amino acid sequence identity. Conserved residues are shaded black. Alpha helices are indicated by black bars and β-sheets are indicated with black arrows. The highly conserved W residue is demarcated with a black asterisk (*)
