Abstract
Objectives
To test the hypothesis that esophageal mechanodistention in infants with hypoxic–ischemic encephalopathy (HIE) results in altered upper esophageal sphincter (UES), esophageal body, and lower esophageal sphincter (LES) responses, compared with controls. As a secondary aim, we tested the hypothesis that infants with HIE receiving therapeutic hypothermia had different aerodigestive reflex characteristics than infants with HIE who received traditional neonatal care.
Study design
Provocative esophageal manometry was performed in 34 neonates (27 with HIE and 7 controls). Mechanodistention was performed using graded volumes of air. Peristaltic reflexes, UES contractile reflexes, and LES relaxation reflexes were analyzed for frequency, magnitude, and aberrancies.
Results
Infants with HIE demonstrated more rapid recruitment of responses and greater UES magnitude (P < .05). They had more frequent secondary peristalsis and lower LES nadir pressures with prolonged LES nadir durations (P < .05). Most notable were the prolonged peristaltic response durations and increases in the number of polymorphic waveforms (P < .05). Compared with infants with HIE receiving traditional care, infants with HIE treated with hypothermia had higher UES pressures and shorter peristaltic response duration (P < .05).
Conclusions
Mechanodistention in infants with HIE results in upregulation of central vagal effects (ie, heightened cholinergic excitatory responses as demonstrated by exaggerated UES contractile reflex activity and heightened inhibitory responses evident by exaggerated LES relaxation reflex activity). Prolonged and poorly coordinated peristaltic responses may underlie dysfunction of aerodigestive regulation. Modulation of sensorimotor aspects of aerodigestive reflexes is altered in infants with HIE, and hypothermia may further modify such effects.
Perinatal asphyxia occurs in 3–5 per 1000 live births and presents as hypoxic–ischemic encephalopathy (HIE), a cause of early brain injury.1 Complications of perinatal asphyxia account for 23% of neonatal deaths worldwide, and survivors may have a variety of neurologic deficits, including cerebral palsy (CP), mental retardation, and epilepsy.1,2 Feeding difficulties, highly associated with neurologic insult3–5 and seen in >50% of the most severely affected,6–8 are also prevalent in HIE. Because feeding and swallowing are the first assessments of overall health and neurodevelopmental well-being in newborns,9 feeding dysfunction may be one of the first indicators of future neurodevelopmental problems. However, the mechanisms of feeding dysfunction are unclear.
Abnormal modulation of the central nervous system and the enteric nervous system has been implicated as an underlying mechanism for abnormal esophageal motility, gastroesophageal reflux (GER), and aspiration in children with neurologic insult.10,11 Perinatal asphyxia causes neuronal necrosis during a critical time of nerve myelination and brain maturation in 3 major regional patterns.12 Whether the pattern of injury is diffuse, cerebral–deep nuclear, or deep nuclear–brainstem, there is potential for significant deficits in neural communication with the oral cavity, posterior pharynx, upper esophageal sphincter (UES), esophageal body, and lower esophageal sphincter (LES).
Esophageal clearance, essential for aerodigestive safety, requires appropriately timed and coordinated peristaltic responses. It is dependent on neuromuscular interaction as well as the volume and physicochemical properties of the stimuli.13 Neuro-muscular responses to esophageal provocation after HIE are poorly understood, and the mechanisms of aerodigestive protection and clearance remain unclear. Furthermore, there is a significant risk of death, disability, aspiration, GER, and chronic feeding problems in infants with HIE.14,15 Therefore, our primary aim was to test the hypothesis that esophageal provocation–induced aerodigestive reflexes are impaired in infants with HIE compared with controls. Because hypothermia is an increasingly prevalent practice and outcomes are reportedly better; as a secondary aim, we tested the hypothesis that infants with HIE receiving therapeutic hypothermia had different aerodigestive reflex characteristics than infants with HIE who received traditional neonatal care.
Methods
Overall, 34 infants were evaluated (27 with HIE and 7 controls). The infants were admitted to Nationwide Children’s Hospital from outlying hospitals. Gestational age (GA) was determined by maternal history, obstetric data, and examination. Postmenstrual age was determined by adding GA to chronologic age. The parents gave informed, written consent. The studies were performed after Nationwide Children’s Hospital Institutional Review Board approvals were obtained, and we complied with the Health Insurance Portability and Accountability Act. Subject safety was monitored throughout the study.
Infants were included in the HIE arm if they: (1) were >36 weeks’ GA; (2) experienced acute perinatal asphyxia defined as an acute perinatal event (placental abruption, cord prolapse, severe fetal heart rate [abnormality]); and (3) presented with signs of encephalopathy per Sarnat staging16 at birth. The traditional HIE group included 10 of 11 infants evaluated before 2010 and 1 infant evaluated in 2011. In addition, 16 infants with HIE received hypothermia treatment between April 2010 and December 2011. All infants had esophageal motility tracings that were appropriate for analysis.
Healthy age-matched full-term controls are a difficult population to recruit for pragmatic and ethical reasons. Therefore, we adopted strict inclusion criteria of choosing control infants who had good oral feeding ability and were thriving in the nursery. These infants ranged from 32–37 weeks’ GA at birth and needed observation in the nursery for prematurity, respiratory distress, or transitional problems. However, the postmenstrual age at the time of the study for the control infants was 39 ± 1 week (within the full-term range). The selection of control infants for esophageal motility studies was maturationally appropriate, as their feeding milestones were appropriate and comparable with those of full-term infants.6 Infants were excluded if they had congenital, genetic, or chromosomal abnormalities. The infants with HIE who received therapeutic hypothermia met established criteria.14
Infants with HIE were compared with healthy controls to ascertain disease effect on UES, esophageal body, and LES characteristics. Similar comparisons were then made between infants with HIE who received traditional care and those who received therapeutic hypothermia. For each HIE group, aero-digestive outcomes were noted and feeding outcomes were collected at discharge and at 1 year.
Manometry Methods
As previously described,17,18 a specially designed manometry catheter with a UES sleeve, pharyngeal port, 3 esophageal ports, an LES sleeve, and a stomach port were connected to a minimally compliant micromanometric water perfusion system (Dentsleeve International; Mui Scientific, Mississauga, Ontario, Canada). The manometric catheter ports were attached to the recording system via resistors (0.01, 0.02, 0.04, and 0.08 mL/min), transducers (Ohmeda TNF-R disposable pressure transducers; Medical Measurement Systems USA, Dover, New Hampshire), and amplifiers (Solar modules, solar-2, Medical Measurement Systems USA).
Manometry Protocol
Studies were performed with the infant supine and the transducers at the level of the subject’s esophagus (midaxillary line). The catheter was passed nasally, and a pull-through technique was used to ensure appropriate positioning as described previously.18 Graded volumes of midesophageal air were infused abruptly in triplicate (0.1, 0.5, 1.0, 2.0, and 5.0 mL) to test the effects of stimulus–response relationships. Each volume was given in the same order during esophageal quiescence and after an interval of at least 1 minute elapsed for esophageal clearance. In addition to pharyngeal manometric waveforms and direct observation or video recording, submental electomyography was used to record the initiation of the pharyngeal phase of swallowing.
Manometry Data Analysis
Swallowing reflexes and esophageal body characteristics were analyzed as defined previously.13,18–21 Secondary peristalsis was defined as the propagation of the peristaltic wave front across the proximal, middle, and distal esophagus in the absence of pharyngeal waveform and UES relaxation. Esophagodeglutition reflex was defined as the deglutition response on esophageal stimulation, which began with onset of the pharyngeal waveform associated with UES relaxation and propagation into the proximal, middle, and distal esophageal segments and associated with LES reflex. The primary response time was noted for each secondary peristalsis and EDR and was defined as the time elapsed from the onset of infusion to the initiation of the swallowing reflex. As described previously,13,20 UES characteristics were analyzed for resting UES pressure, maximal UES pressure after infusion peak, and UES contractile reflex. LES characteristics were analyzed for resting LES pressure, nadir LES pressure, and duration of LES nadir as described previously.18 Multiple esophageal body waveforms noted in the proximal, middle, or distal esophagus during an EDR or secondary peristalsis were defined as polymorphic waveforms. These waveforms occurred within a single segment, in multiple segments, or simultaneously between segments during peristalsis. The duration of esophageal body peristaltic response was defined as the period between the onset of the first peristaltic waveform until the offset of the last peristaltic waveform occurring in the proximal, middle, and/or distal esophageal segments.
Statistical Analyses
Esophageal reflexes were identified by 2 trained observers at the time of the evaluation and the data were evaluated using the criteria described earlier. Mixed statistical models were applied for the comparisons when reporting UES, esophageal body, and LES characteristics. Polymorphic waveforms and propagation of peristalsis were evaluated using χ2 test and multiple logistic regression with PROC GENMOD (SAS Institute, Cary, North Carolina). Mean ± SE values are reported, unless stated otherwise, and adjusted values of P < .05 were considered statistically significant. Power analysis showed that when analyzing the differences in esophageal reflexes between groups, the probability of Type I error of our analysis is 5% (α = .05), and the probability of Type II error is <1%. In this case, the analyses we used in the current study carried both high sensitivity and high specificity.
Results
Postmenstrual age at the time of the study for the infants with HIE was 42 ± 0.5 week, and for the control infants, it was 39 ± 1 week (P = .2). Growth characteristics (weight, length, and head circumference) for the control and HIE groups were similar. At our institution, antiepileptic drugs are initiated if seizures are noted clinically or on electroencephalogram. Twenty-two of 27 infants with HIE were receiving maintenance phenobarbital, levetiracetam, or both at the time of their study. As a subgroup analysis within the HIE category, we describe the demographic and neurologic characteristics (Table I) and the aerodigestive interventions and milestones (Table II).
Table I.
Demographic and neurological characteristics of HIE infants, hypothermia versus traditional care
| Characteristic | Hypothermia (16) | Traditional care (11) | P |
|---|---|---|---|
| Male, No. (%) | 8 (50) | 4 (36) | .7 |
| GA, wk, mean ± SE | 38 ± 0.4 | 39 ± 0.5 | .3 |
| Birth weight, g, mean ± SE | 3344 ± 219 | 3674 ± 263 | .3 |
| Head circumference, cm, mean ± SE | 34 ± 0.5 | 35 ± 0.5 | .3 |
| Apgar score at 1 min, mean (range) | 1 (0–3) | 1(0–9) | .5 |
| Apgar score at 5 min, mean (range) | 4 (0–7) | 1 (0–9) | .4 |
| Sarnat stage 2, No. (%) | 11 (69) | 6 (55) | .7 |
| Sarnat stage 3, No. (%) | 5 (31) | 5 (45) | .7 |
| Initial EEG abnormality, No. (%) | 15 (94) | 8 (80)* | .5 |
| Electrographic seizure, initial EEG, No. (%) | 5 (31) | 6 (60)* | .2 |
| Clinical seizure, No. (%) | 8 (50) | 8 (80) | .2 |
| MRI findings of HIE, No. (%) | 11 (69) | 11 (100) | .06 |
| Basal ganglia-thalamic abnormality | 9 (56) | 10 (91) | .09 |
| Brainstem abnormality | 4 (25) | 2 (18) | .7 |
| Other abnormal findings† | 4 (25) | 3 (27) | .9 |
| Normal MRI | 1 (6) | 0 | 1.0 |
EEG, electroencephalography; MRI, magnetic resonance imaging.
EEG not available for 1 patient.
MRI findings other than HIE changes included hemorrhage, volume loss, or fluid collections.
Table II.
Aerodigestive interventions and milestones of HIE infants, hypothermia versus traditional care
| Aerodigestive interventions and milestones | Hypothermia (16) | Traditional care (11) | P |
|---|---|---|---|
| Airway, d, mean ± SE | |||
| Ventilator | 14 ± 5 | 7 ± 3 | .2 |
| NCPAP | 3 ± 1 | 8 ± 3 | .05 |
| Nasal cannula | 11 ± 3 | 18 ± 4 | .2 |
| Room air | 19 ± 4 | 29 ± 5 | .1 |
| Feeding, mean ± SE | |||
| TPN/IL, d | 19 ± 2 | 13 ± 1 | .01 |
| First feed, DOL | 7 ± 0.6 | 6 ± 0.5 | .08 |
| Full enteral feeds, DOL | 19 ± 2 | 14 ± 1 | .03 |
| Full PO, DOL | 29 ± 6 | 34 ± 6 | .7 |
| Full PO, No (%) | 7 (44) | 3 (27) | .4 |
| Additional interventions | |||
| Tracheostomy, N (%) | 2 (13) | 2 (18) | .7 |
| Gastrostomy, N (%) | 9 (56) | 8 (73) | .4 |
DOL, day of life; IL, intra lipid; NCPAP, nasal continuous positive airway pressure; PO, oral feed; TPN, total parenteral nutrition.
At discharge, exclusive oral feeding was achieved in 10 infants with HIE (37%). Within the HIE group, subgroup analysis revealed that oral feeding was noted in 44% of the hypothermia group versus 27% of the traditional group (P = .5). At 1 year of age, 22 of 27 infants with HIE had feeding outcomes documented (1 lost to follow-up, 1 died, and 3 <1 year of age). Among these, oral feeding was observed in 75% of the hypothermia infants versus 30% of the traditional care infants (P = .08).
Three main responses to midesophageal distention were identified: (1) peristaltic reflexes; (2) UES contractile reflex; and (3) LES relaxation reflex. Provocation with air stimuli resulted in 70% peristatic reflexes, 53% UES contractile reflex, and 64% LES relaxation reflex in the HIE group and 55% peristatic reflexes, 57% UES contractile reflex, and 52% LES relaxation reflex in the control group. In comparing the groups, significance with esophageal air-induced reflexes was noted for peristatic reflexes (P = .007) and LES relaxation reflex (P = .04). Frequency of polymorphic waveforms (HIE 23%, control 6%) and secondary peristalsis (HIE 57%, control 44%) was significant (P = .003 and .03, respectively). Response time to peristaltic reflexes (HIE: 3.4 ± 0.1 second vs control: 4.3 ± 0.3 second; P = .02) and UES contractile reflex (HIE: 2.1 ± 0.1 second vs control: 3.3 ± 0.4 second; P = .006) occurred more quickly in infants with HIE. Similarities in proportion of responses were noted in the traditional and hypothermia groups.
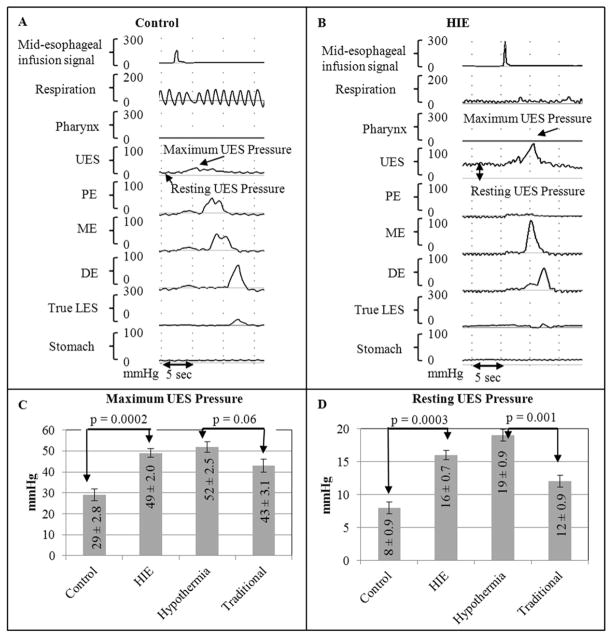
Resting UES pressures and maximal rise in UES pressures were noted to be greater in the HIE group compared with the control group. Furthermore, these pressures remained greater in the hypothermia group compared with the traditional group (Figure 1).
Figure 1.
UES on midesophageal distention in A, controls and B, infants with HIE. C, Maximum UES pressure and D, resting UES pressure were both noted to be significantly greater in infants with HIE compared with controls, and these pressures remained consistently greater in infants who received hypothermia compared with infants who received traditional care. DE, distal esophagus; ME, middle esophagus; PE, proximal esophagus.
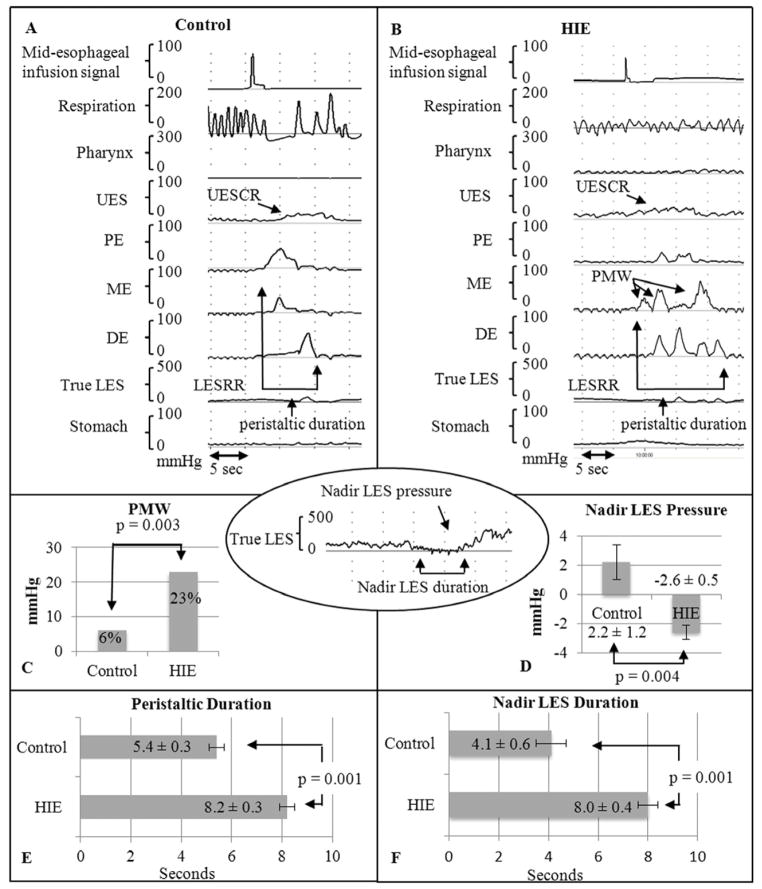
The most notable differences in infants with HIE consisted of prolonged peristaltic response duration after esophageal provocation and a significant increase in the number of polymorphic waveforms (Figure 2).
Figure 2.
Esophageal characteristics on midesophageal distention in A, controls and B, infants with HIE. Remarkable differences in HIE were noted with C, polymorphic waveform frequency, D, lower nadir LES pressure, E, peristaltic duration, and F, LES duration. PMW, polymorphic waveform.
Nadir LES pressure was lower and the duration of the LES nadir was significantly longer for infants with HIE compared with controls (Figure 2).
No significant differences in threshold volumes between HIE and control or hypothermia and traditional care were observed. ORs (95% CI) for dose–response relationships, defined as every one unit volume increase would cause the odds of the response to increase by the shown amount, are as follows: (1) for UES contractile reflex, OR was 1.51 (95% CI 1.19–1.90; P < .05) in HIE, in contrast to OR of 1.46 (1.19–1.79; P < .05) for controls; (2) for peristatic reflexes, OR was 2.15 (1.22–3.81; P < .05) in HIE and OR was 1.56 (1.00–2.48; P = .05) for controls; and (3) for LES relaxation reflex, OR was 1.44 (1.09–1.90; P < .05) for HIE and OR was 1.24 (0.93–1.66; P = .1) for controls. Comparisons between the groups showed significant relationship only for the recruitment of LES relaxation reflex (P < .05), and not for UES contractile reflex or peristatic reflexes (P = NS).
Discussion
Using an experimental design and provocative interrogation of esophageal motility reflexes, we investigated the effect of esophageal mechanodistention in infants with HIE. Our working hypothesis was that HIE modifies esophageal reflexes responsible for esophageal clearance, as infants with HIE frequently have aerodigestive problems. We demonstrated significant esophageal dysmotility mechanisms between typically developing infants and infants with HIE. Importantly, peristaltic reflexes, UES contractile responses, esophageal body coordination and clearance, and LES relaxation responses were all noted to be aberrant. Additionally, we found that infants with HIE treated with hypothermia showed greater UES contractile responses and improved esophageal clearance as noted by decreased peristaltic durations.
The HIE group clearly had more difficulty with oral secretion management, feeding coordination, and respiratory issues, as evidenced by the degree of aerodigestive support during hospitalization. The controls were distinct from the infants with HIE as they fed well at discharge and had a normal neonatal course. The aerodigestive problems in the HIE group may be related to impaired regulation and coordination resulting in ineffective clearance mechanisms. Although sensitivity is preserved, the motor responses were prolonged and of greater magnitude.
Mechanosensitive esophageal provocation typically activates upstream protective reflexes such as UES contractile reflex13,19 and downstream protective reflexes such as LES relaxation reflex.18 In addition, esophageal clearance is achieved through peristaltic reflexes such as secondary peristalsis and EDR. Thus, stimulus-related activation of appropriate upstream and downstream motor events result in clearance and aerodigestive tract protection. Prolonged peristaltic durations, more rapid responses to mechanosensitive provocation, and heightened sphincter responses were noted in the infants with HIE. These findings may be related to increased cholinergic activation, resulting in: (1) more rapid and frequent UES responses; (2) increased UES contractile activity; (3) increased sensitivity of the esophagus during peristalsis; and (4) increased inhibition of LES function resulting in heightened relaxation and prolongation of responses.
Ischemia–reperfusion injury, the basis of HIE, also has potential to disrupt brainstem regulatory function mediated by the vagus nerve. Dysfunction may occur through alteration of acetylcholine, adenosine triphosphate, nitric oxide, or other neurotransmitter release and interaction at the muscular level.22 This altered interaction may have modified the excitatory and inhibitory effects required for aerodigestive function and protective reflexes.
Regulating neuromotor and inflammatory pathways requires a balance between acetylcholine activity and acetylcholinesterase activity. Impaired cholinergic signaling has been implicated in severe inflammatory states,23 as well as a variety of diseases that are largely acquired either through aging, either acute stress or, with environmental toxic exposure. A range of cholinergic agonists and antagonists to remediate these inflammatory and neuromuscular diseases have been proposed.24–27
Our data suggest increased cholinergic excitatory activity as the basis for: (1) the increased UES contractile responses; (2) increased sensitivity of the esophagus; and (3) increased cholinergic inhibitory activity resulting in heightened LES relaxation response in infants with HIE. Exact delineation of these pathways is not feasible in human models and not entirely clear from animal models. Because HIE results in diffuse systemic inflammation similar to that seen in other disease, a cholinergic anti-inflammatory response may result from activation of similar pathways.28 Given that esophageal motility functions are regulated by parasympathetic pathways, the upregulation of cholinergic excitatory responses (eg, rapid and increased frequency of UES contractile response, more frequent secondary peristalsis) and the upregulation of cholinergic inhibitory responses mediated via nitric oxide or vasoactive intestinal polypeptide pathways at the LES level may occur (eg, greater decrease in LES nadir pressure, prolonged LES nadir duration). The functional significance of such responses is not clear, although hypersensitivity and hyperreactivity to visceral stimuli may be plausible explanations, and such responses are protective in thwarting and clearing stimuli away from the aerodigestive tract, such as may happen during GER events. In patients with progression of lesions associated with HIE, the clinical picture can be different with dysphagia, GER disease, and aerodigestive sequelae. Further studies are needed to clarify longitudinal changes in infants with HIE.
During the past decade, there have been trials in infants with HIE comparing therapeutic hypothermia with traditional therapy. The protective effects of hypothermia are proposed to be: (1) reduction in cellular metabolism and oxygen demand while maintaining adenosine triphosphate levels; (2) attenuation of abnormal free radical production; and (3) improvement of cell ion handling and improvement in pH balance. The overall effect being reduced cell death and inflammatory signaling.22 A recent meta-analysis of the largest trials14,29,30 strongly supports the use of therapeutic hypothermia in HIE to reduce death and neurologic impairment at 18 months.15 In these trials, neurologic impairment is defined on the basis of survival with or without CP, mental developmental index scores, psychomotor development index scores, vision abilities, and hearing abilities. The meta-analysis does not specifically address the mechanisms of swallowing and feeding (dis)abilities in HIE. Our data show that infants with HIE had elevated resting and maximal UES pressures compared with controls, and this response was further elevated in infants who received therapeutic hypothermia. If a cholinergic anti-inflammatory response is a protective pathway against inflammation and neuromotor injury, this may suggest that hypothermia adds further protection to the disease process in HIE. Preservation of these interactions via therapeutic hypothermia may improve brainstem-mediated reflexes in infants with HIE. Further studies are needed to address the effect of therapeutic hypothermia on aerodigestive functions at multiple levels. Furthermore, to evaluate the changes in neuromotor responses with time, serial longitudinal studies are necessary.
Impaired reflexes can also be due to antiepileptic medications, although it is unlikely the major reason. In our study, the UES contractile reflex frequency and magnitude were both high. On the contrary, in those overly sedated, hypotonia is more common. Generally, when subjects are on maintenance antiepileptic doses, they are able to maintain normal biological reflexes (eg, sucking, swallowing, breathing, gastric emptying, defecation) and, indeed, all infants in the current study were tolerating enteral feeds at study. One infant in the hypothermia group and 3 infants in the traditional group required no antiepileptic medication. Infants were physiologically stable at evaluation from a nutrition, feeding, and seizure standpoint.
The incidence of HIE lesions on magnetic resonance imaging between the groups is also of interest. We will need further observations to clarify associations. Basal ganglia abnormalities were more evident in the traditional group, and this area is an important center for regulation and coordination of muscular tone, activity, and feeding.
The implications of this study are several. It is commonly speculated that HIE and other neurologic injury interferes with central and enteric nervous system communication with potential for significant aerodigestive maladaptation affecting safety and function. Our study supports this concept. It was recently recognized that feeding difficulties in children with fully developed CP are associated with inadequate nutritional intake, resulting in poor general health, suboptimal fat deposition, and poor growth. The neuromotor dysfunction in infants with HIE places them at risk for aerodigestive problems. With this in mind, many studies, surgeries, and medications may put them at additional risk given the lack of proved therapies for esophageal or aerodigestive malfunctions. Furthermore, procedures such as gastrostomy or fundoplication, although frequently performed, do not account for underlying dysmotility.10
As noted with children with CP, we have shown that neuromotor interaction at the esophageal level is aberrant and development in HIE may proceed through dysfunctional afferent and efferent motor responses.5 These motor patterns, when built on dysfunctional neuronal reflexes and a possibly deficient neuronal pool, produce learned responses that may not be adequate for safe and effective oral feeding. Based on the current study, UES hypertonia and prolonged contractility may be an early therapeutic target. Hypothermia as therapy will require further longitudinal studies to determine if there is a protective role early that later improves swallowing and aerodigestive safety. It is encouraging that our study found that infants receiving hypothermia had significant oral feeding skills at 1 year. Longitudinal changes in UES and LES functions in infants with HIE will also need further investigation.
Acknowledgments
The authors are thankful to Yue Jin, MS, for statistical assistance. They also thank the Scholarship Oversight Committee members (Ed Shepherd, MD, Warren Lo, MD, and Mark Splaingard, MD) for their insight during C.H.’s training.
Supported in part by the National Institutes of Health (R01 DK 068158 to S.J.).
Glossary
- CP
Cerebral palsy
- EDR
Esophago-deglutition response
- GA
Gestational age
- GER
Gastroesophageal reflux
- HIE
Hypoxic–ischemic encephalopathy
- LES
Lower esophageal sphincter
- UES
Upper esophageal sphincter
Footnotes
The authors declare no conflicts of interest.
References
- 1.Gonzalez FF, Ferriero DM. Neuroprotection in the newborn infant. Clin Perinatol. 2009;36:859–80. doi: 10.1016/j.clp.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J for the Lancet Neonatal Survival Steering Team. 4 Million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Del Giudice E, Staiano A, Capano G, Romano A, Florimonte L, Miele E, et al. Gastrointestinal manifestations in children with cerebral palsy. Brain Dev. 1999;21:307–11. doi: 10.1016/s0387-7604(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 4.Otapowicz D, Sobaniec W, Okurowska-Zawada B, Artemowicz B, Sendrowski K, Kulak W, et al. Dysphagia in children with infantile cerebral palsy. Adv Med Sci. 2010;55:222–7. doi: 10.2478/v10039-010-0034-3. [DOI] [PubMed] [Google Scholar]
- 5.Gisel E. Interventions and outcomes for children with dysphagia. Dev Disabil. 2008;14:165–73. doi: 10.1002/ddrr.21. [DOI] [PubMed] [Google Scholar]
- 6.Jadcherla S, Wang M, Vijayapal A, Leuthner S. Impact of prematurity and co-morbidities on feeding milestones in neonates: a retrospective study. J Perinatol. 2010;30:201–8. doi: 10.1038/jp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Biarge M, Diez-Sebastian J, Rutherford MA, Cowan FM. Outcomes after central grey matter injury in term perinatal hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86:675–82. doi: 10.1016/j.earlhumdev.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Jadcherla SR, Shaker R. Esophageal and upper esophageal sphincter motor function in babies. Am J Med. 2001;111:64S–8S. doi: 10.1016/s0002-9343(01)00848-8. [DOI] [PubMed] [Google Scholar]
- 9.Delaney AL, Arvedson JC. Develpment of swallowing and feeding: pre-natal through first year of life. Dev Disabil. 2008;14:105–17. doi: 10.1002/ddrr.16. [DOI] [PubMed] [Google Scholar]
- 10.Pensabene L, Miele E, DelGiudice E, Strisciuglio C, Staiano A. Mechanisms of gastroesophageal reflux in children with sequelae of birth asphyxia. Brain Dev. 2008;30:563–71. doi: 10.1016/j.braindev.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Andrew MJ, Parr JR, Sullivan PB. Feeding difficulties in children with cerebral palsy. Arch Dis Child Educ Pract Ed. 2012;97:222–9. doi: 10.1136/archdischild-2011-300914. [DOI] [PubMed] [Google Scholar]
- 12.Volpe J. Neurology of the newborn. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 13.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr. 2006;149:77–82. doi: 10.1016/j.jpeds.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 15.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;240:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarnat H, Sarnat M. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 17.Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr. 2007;151:597–603. doi: 10.1016/j.jpeds.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pena EM, Parks VN, Peng J, Fernandez SA, Di Lorenzo C, Shaker R, Jadcherla SR. Lower esophageal sphincter relaxation reflex kinetics: effects of peristaltic reflexes and maturation in huma premature neonates. Am J Physiol Gastrointest Liver Physiol. 2010;299:1386–95. doi: 10.1152/ajpgi.00289.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–8. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 20.Jadcherla SR, Parks VN, Peng J, et al. Esophageal sensation in premature human neonates: temportal relationsips and implications of aerodigestive reflexes and electrocortical arousals. Am J Physiol Gastrointest Liver Physiol. 2012;302:134–44. doi: 10.1152/ajpgi.00067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophagoglottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol. 2007;102:2286–93. doi: 10.1111/j.1572-0241.2007.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lampe JW, Becker LB. State of the art in therapeutic hypothermia. Annu Rev Med. 2011;62:79–93. doi: 10.1146/annurev-med-052009-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadcherla SR. Inflammation inhibits muscarinic signaling in in vivo canine colonic circular smooth muscle cells. Pediatr Res. 2002;52:756–62. doi: 10.1203/00006450-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Ofek K, Soreq H. Cholinergic involvement and manipulation approaches in multiple system disorders. Chemicobiol Interact. 2012 doi: 10.1016/j.cbi.2012.07.007. In press. [DOI] [PubMed] [Google Scholar]
- 25.Tabet N. Acetylcholinesterase inhibitors for Alzheimer’s disease: anti-inflammatories in acetylcholine clothing! Age Ageing. 2006;35:336–8. doi: 10.1093/ageing/afl027. [DOI] [PubMed] [Google Scholar]
- 26.Borovilova L, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 27.Ofek K, Krabbe K, Evron T, Debecco M, Nielsen AR, Brunnsgaad H, et al. Cholinergic status modulations in human volunteers under acute inflammation. J Mol Med. 2007;85:1239–51. doi: 10.1007/s00109-007-0226-x. [DOI] [PubMed] [Google Scholar]
- 28.Gallowitsch-Puerta M, Pavlov V. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007;80:2325–9. doi: 10.1016/j.lfs.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 30.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]