Abstract
Inshore and offshore waters of the Gulf of Maine (USA) have spring/summer harmful algal blooms (HABs) of the toxic dinoflagellate Alexandrium fundyense, which is responsible for paralytic shellfish poisoning (PSP) in humans. The calanoid copepod Calanus finmarchicus co-occurs with A. fundyense during the seasonal blooms. At that time, C. finmarchicus population abundances are high, dominated by immature copepods preparing for diapause, and by actively-reproducing adults. High survival has been reported for copepods exposed to toxic A. fundyense, but little is known about possible sublethal effects. In this study, C. finmarchicus adult females were fed either a control diet of non-toxic Rhodomonas spp. or one of two diets containing either low dose (LD) or high dose (HD) levels (50 and 200 cells mL−1, respectively) of toxic A. fundyense for a total of 7 days in two independent experiments. As expected, ingestion of the dinoflagellate had no effect on copepod survival and grazing activity. However, significant reductions of egg production and egg viability were observed in C. finmarchicus females fed on either experimental diet. After the 7-day experiment, total nauplius production by females on the LD and HD diets was reduced by 35% to 75% compared to the control females. These results suggest that blooms of A. fundyense in the Gulf of Maine may be an environmental challenge for C. finmarchicus populations, with a potential negative effect on copepod recruitment.
Keywords: Calanus finmarchicus, sublethal effects, Alexandrium fundyense, saxitoxins, Gulf of Maine
1. INTRODUCTION
Harmful algal blooms (HABs) dominated by the dinoflagellate Alexandrium fundyense occur annually in offshore and inshore waters of the Gulf of Maine (Anderson et al., 2005; 2014; Martin and White, 1988; Shumway et al., 1988;). Although blooms are highly variable both spatially and temporally, dense regional blooms (103–104 cells L−1) of A. fundyense occur every summer (Anderson, 1997; Deeds et al., 2014; McGillicuddy et al., 2014). By producing potent neurotoxins, known as saxitoxins (STXs), A. fundyense is responsible for outbreaks of paralytic shellfish poisoning (PSP), which is potentially fatal to humans (Llewellyn, 2006). Although STXs are highly toxic to most vertebrates, numerous invertebrate herbivores, such as copepods and shellfish are able to ingest the toxic algae without affecting their survival (Bricelj and Shumway, 1998; Petitpas et al., 2014; Shumway, 1990; Teegarden et al., 2003). These invertebrates retain and accumulate toxins in their tissues, becoming vectors for transfer to higher trophic levels, including fishes, whales and humans (Anderson and White, 1992; Campbell et al., 2005; Doucette et al., 2005; 2006; Petitpas et al., 2014; Turner and Tester, 1997). It is less clear whether and how A. fundyense affects the overall fitness of the invertebrate filter feeders (Turner, 2014).
Calanus finmarchicus is one of the more abundant calanoid copepods in the North Atlantic, extending from the mid-Atlantic Shelf off the US east coast to the Barents Sea north of Norway (Conover, 1988; Planque et al., 1997). In the Gulf of Maine, the C. finmarchicus population increases during the spring (Davis, 1987; Meise and O’Reilly, 1996) with the copepod serving as food for planktivorous fishes such as larval herring and mackerel (Darbyson et al., 2003). During the summer, when A. fundyense blooms are present, C. finmarchicus can dominate the zooplankton biomass (Davis, 1987; Sherman et al., 1987), sometimes reaching abundances of 10,000 m−3 or higher (Meise and O’Reilly, 1996). During this period the C. finmarchicus population is dominated by mid- and late-stage copepodites (Durbin et al., 2000; Miller et al., 1998). Not surprisingly, during bloom conditions C. finmarchicus and other zooplankters ingest A. fundyense, as confirmed in several field studies (Campbell et al., 2005; Turner, 2006; 2010; Turner and Borkman, 2005; Turner et al., 2005). Survival in these studies as well as laboratory grazing studies was high even when A. fundyense was the only food (Teegarden et al., 2001; 2008; Hassett, 2003). However, previous grazing studies were mostly limited to incubations of 24 h or less (Hassett, 2003; Teegarden et al., 2001; 2008; Turner, 2010; Turner and Borkman, 2005). In addition, none of these studies investigated the possibility of other effects on C. finmarchicus (Turner, 2014).
Studies on other copepods have shown that Alexandrium spp. can have adverse effects such as low feeding rates, low egg production and low egg hatching success (Colin and Dam, 2007; Dutz, 1998; Guisande et al., 2002; Sopanen et al., 2011; Teegarden et al., 2008; Turner, 2014). Reduced feeding rates have been measured for Acartia tonsa and Eurytemora herdmani fed on A. fundyense at very high concentrations (ca. 500–2000 cell mL−1) (Teegarden and Cembella, 1996), while Acartia clausi responded to exposure to Alexandrium spp. with decreased growth and fecundity, suggesting either reduced caloric intake (low feeding activity) reduced food assimilation or possible reallocation of energy into detoxification (Dutz, 1998; Frangópulos et al., 2000; Guisande et al., 2002). Overall, these sublethal effects, induced by Alexandrium spp. in different copepod species, suggest that the presence of the toxic dinoflagellate could be a significant environmental stressor. Thus, HABs could disrupt existing pelagic communities in ways that are difficult to predict based on studies focused solely on survivorship.
The goal of the present study was to determine whether A. fundyense might have a negative effect on C. finmarchicus fitness. Adult female survival, feeding rates and reproductive success were monitored during 7-day-long experiments in which the animals were maintained on one of three different diets: control with no A. fundyense, low dose (LD) with (25:75 by cell volume A. fundyense and Rhodomonas sp.) and high dose (HD) with 100% A. fundyense. The experimental algal concentration for the LD was comparable to bloom conditions in the Gulf of Maine (Anderson, 1997; McGillicuddy et al., 2014; Petitpas et al., 2014).
2. MATERIALS AND METHODS
2.1. Field collection and maintenance of Calanus finmarchicus
Calanus finmarchicus were collected in June and July 2012 in the Gulf of Maine near Mount Desert Rock (Lat: 44° 2′N; Long: 68°3′W) by slowly towing a 75 cm diameter (560 μm mesh) net vertically from 75 m depth to the surface. Plankton collections were immediately diluted into buckets containing 10 L of subsurface seawater and placed on ice in coolers for transportation to the Mount Desert Island Biological Laboratory (Salisbury Cove, ME) for experiments (generally within 3 hours of collection). Healthy adult females and adult males were sorted from the diluted plankton samples and transferred into 3.5 L jars of filtered seawater (FSW) with 15–20 individuals per jar (1:3 male and females) with Rhodomonas sp. added ad libitum, and placed overnight into an incubator (Percival Model I-36VL, Percival Scientific, Inc., Perry, IA, USA), maintained at 10°C on a 14:10 h light:dark cycle.
2.2. Experimental design
Calanus finmarchicus adult females were fed with the toxic A. fundyense over a 7-day period; one experiment was performed in June and another in July (Table 2). The experimental design included three treatments: control, low dose (LD) and high dose (HD) diets of A. fundyense (Table 1). The control group consisted of a unialgal diet of the non-toxic flagellate Rhodomonas sp. (8000 cells mL−1), which does not appear to impair copepod egg production and hatching success and is routinely used for maintenance of copepods in culture settings (Helland et al., 2003). The LD treatment consisted of a mixed diet of A. fundyense (50 cells mL−1) and Rhodomonas sp. (6000 cells mL−1), which corresponded approximately to a 1:3 proportion by algal volume. The HD treatment corresponded to 100% of A. fundyense at a concentration of 200 cells mL−1. Although nutritional profiles can be variable, comparable lipid and amino acid profiles have been reported for Rhodomonas spp. and A. fundyense (Ianora et al., 2004; Seixas et al., 2009). Equivalent carbon concentrations were computed for each treatment for Rhodomonas sp. and A. fundyense based on measurements of carbon to volume relationships from Menden-Deuer and Lessard (2000). Estimated carbon concentrations for the experimental food levels were similar to each other, albeit slightly higher in the HD treatment (Table 1). Dinoflagellate toxin content (= toxin cell quota) can vary by a factor of 2 or more within a period of 24 hours (Anderson et al. 1990). Therefore, during the duration of the June and July experiments A. fundyense samples were collected daily for toxin content analysis (see section 2.3, Table 2).
Table 2.
Summary of parameters monitored in adult female Calanus finmarchicus during 7-day experiments in June and July 2012
| Conditions | WHOI | 200 mL containers | 100 mL containers | ||||
|---|---|---|---|---|---|---|---|
| # females | -- | 2 per container | 1 per container | ||||
| Measurements | Algal toxicity | Survival | Grazing rate* | Survival | Fecal pellet production | Egg production | Egg viability |
| June | X | X | - | X | X | X | X |
| July | X | X | X | X | X | X | X |
Grazing rate was measured as clearance and ingestion rate
WHOI= Woods Hole Oceanographic institution
Table 1.
Experimental treatments: daily food added to C. finmarchicus adult females kept at 1 female per 100 mL during two 7-day experiments
| Treatment | |||
|---|---|---|---|
| Control | Low dose | High dose | |
| 100% Rhodomonas spp. | 25Alex: 75 Rho | 100% A. fundyense | |
| Cell mL−1 | 8000 | Rho=6000 | 200 |
| Alex=50 | |||
| Total=6050 | |||
| μg C L−1 | |||
| 304* | Rho=228* | 358** | |
| Alex**=89 | |||
| Total=317 | |||
Each treatment had the same total volume. Carbon volume relationship has been calculated using the formula from Menden-Deuer and Lessard (2000).
Carbon content for for Rhodomonas baltica (38 pgC cell−1) (Fields et al., 2015)
Carbon content estimated for A. fundysense (1790 pgC cell−1). Cell volume has been calculated using formula shape-2 from (Sun and Liu, 2003).
For both the June and July experiments C. finmarchicus adult females were maintained at a density of 1 female per 100 mL with fresh food added daily for each treatment over the 7-day period. At the beginning of each day the animals were transferred from the previous day’s incubation container to a new one. During each experiment (June and July) several C. finmarchicus parameters were monitored: survival rate, egg production rate, egg viability, fecal pellet production and naupliar production (see section 2.4 and 2.5). In the July experiment, in addition to the aforementioned parameters, C. finmarchicus grazing activity was measured.
2.3. Phytoplankton and A. fundyense toxin profile
The flagellate Rhodomonas sp. was used for the control diet in both experiments, however, the clones differed between June and July. The Rhodomonas sp. clone (LOT#120406) used in June was obtained from ALGAGEN LLC with a starting volume of 4 L that had an initial cell density of 106 cells mL−1. Cell densities during the experimental week ranged from 0.8 × 106 to 1.6 × 106 cells mL−1 in the stock culture. The Rhodomonas sp. clone (CCMP739) used in July was obtained from NOAA/NMFS (Milford, CT, USA, isolated in 1951 by R. Lasker) with an initial cell density of 106 cells mL−1 in cultures of 4L. During the experiment, cell densities in the stock culture ranged from 1 × 106 to 2 × 106 cells mL−1. For both Rhodomonas sp. clones, cultures were maintained at 15–16 °C in ambient outside natural light and diluted by 50% with f/2 medium every three days (Guillard, 1973). The toxic dinoflagellate A. fundyense (clone GTCA28) was isolated from the western Gulf of Maine in 1985 and maintained at 15 °C on a 14:10 h light:dark cycle. One-liter cultures were initially grown in modified f/2-Si medium (Anderson et al., 1994) at 15 °C and then transferred to 10 °C during mid-exponential growth for temperature equilibration prior to shipment from Woods Hole, MA to Mount Desert island, ME. There, the cultures were maintained at 10 °C on a 14:10 h light:dark and diluted by 50% every two days with f/2-Si medium (Guillard, 1973). During the experiments, cell densities in the stock cultures ranged from 17 × 103 to 32 × 103 cells mL−1 in June and 14 × 103 to 19 × 103 cells mL−1 in July. Stock cultures of Rhodomonas spp. and A. fundyense were checked 3 times per week between experiments and daily during experiments to assure that cells looked healthy and were swimming actively.
Food suspensions for the experimental treatments were prepared daily by diluting algal cells from the stock cultures into filtered seawater (FSW) at the target concentrations shown in Table 1. For the toxin analysis, three replicate samples of A. fundyense cells were obtained from the stock culture (1.5 mL per sample), transferred into Eppendorf tubes, centrifuged for 8 minutes at 3000 rcf and the supernatant removed. Subsequently, 0.5 mL of 0.05 M acetic acid was added to the pellet and each sample was homogenized using a pipette tip. The sample was shaken twice, stored immediately at 4°C and transported on ice to Woods Hole Oceanographic Institution where the samples were sonicated in an ice water bath using a Branson Sonifier 250 D fitted with a micro-tip probe at a constant 40-watt output for 1 minute. This extract was then stored at − 20 °C. Prior to analysis, the samples were thawed, mixed and centrifuged for 10 minutes at 3000 rcf, and 200 μL of the supernatant was added to a limited volume autosampler vial. Toxin analyses were carried out using a modification of the Oshima (1989) post-column derivatization HPLC method (Anderson et al., 1994). Certified reference standard solutions purchased from the National Research Council Canada (NRC - Halifax, Nova Scotia, Canada), containing toxins C1, C2, GTX1–5, dcGTX2, 3, NEO, dcSTX and STX were run at the beginning of the sample queue and following every 4th sample. The resulting toxin content values were expressed in micromolar concentrations and these were used to calculate cellular toxin content in duplicate or triplicate measurements for each experimental time point. Abbreviations used through this text are: STX= saxitoxin; NEO= neosaxitoxin; GTX1, 4= gonyautoxins 1 and 4; GTX2, 3= gonyautoxins 2 and 3; dcGTX2&3= decarbamoyl gonyautoxin 2 and 3; GTX5= gonyautoxin 5 (or B1, Hall 1982); C1&2 = toxins C1 and C2; dcSTX= decarbamoyl saxitoxin. The concentrations of toxins GTX1&4, GTX2&3 and C1& 2 were combined to account for possible epimerization of the toxin pairs. Toxicities (in STX equivalent cell−1) were calculated from molar composition data using individual potencies provided by the NRC and the daily toxicity level was calculated following (Anderson et al., 1990). The calculation was based on the concentration of individual toxins and their specific toxicity in μg STX eq. μmol−1 as follows: C1, 2.61; C2, 41.6; GTX1, 429.4; GTX2, 155.2; GTX3, 275.6; GTX4, 313.7; GTX5, 27.8; dcGTX2, 66.5; dcGTX3, 162.7; NEO 399.3; dcSTX, 221.7; STX, 432.0.
2.4. Egg production, fecal pellet production, egg viability and naupliar production
Thirty Calanus finmarchicus mature females were randomly selected from the stock jars after overnight acclimation (see above) and incubated individually in 100 mL tissue flasks in one of the three treatment food suspensions, and maintained with the same treatment for the next 7 days. The experiment was performed in June and then replicated in July (Table 2). Every day, each female was transferred with a pipette into a new container, and supplied with a fresh food suspension. The jars from the previous 24 hours containing eggs and fecal pellets produced by each female were incubated for another 24 hours in the 10 °C incubator and then were preserved in 70% ethanol. The contents of the jars were then checked under a dissecting microscope and hatched nauplii, unhatched eggs and fecal pellets were counted. The mass of each fecal pellet was estimated to be the same among the different treatments based on size. Nauplii were checked for possible malformations (none were found). Eggs were also checked for evidence of cannibalism (crumpled egg membrane, none were found).
2.5. Grazing activity
Daily grazing experiments were performed during the July experiment. Nine sets of two mature and healthy C. finmarchicus adult females were randomly selected from the stock jars (see section 2.1) and transferred into crystallizing dishes with 200 mL of one of the three treatment diets. Each treatment consisted of three replicates with food suspensions prepared daily by performing cell counts in stock cultures and adjusting the concentration by diluting with filtered sea water (GF/C Whatman filters). Each day females were transferred into new containers with new experimental food suspensions. For each treatment two additional containers without copepods: a grazing control and an initial were prepared; the initial, representative of daily food suspensions was preserved with Lugol’s solution (2%) at the beginning of each daily experiment while the grazing control was treated like the experimental jars and incubated at 10 °C on a 14:10 h light:dark cycle at 50 μm m−2s−1. After a 22.5–24 hour incubation period, and the transfer of the females to new food suspensions, the contents of the containers (1 grazing control and 3 experimental per treatment) were preserved in 2% Lugol’s solution. Phytoplankton cell numbers in preserved aliquots were counted in Sedgwick-Rafter cells, with a minimum of 400 cells counted in all cases, assuring ca. 10% precision (Guillard, 1973). Computed ingestion and clearance rates of C. finmarchicus were determined from differences in phytoplankton cell concentrations in initial, grazing control and experimental suspensions using the formulae described by Frost (1972). Briefly, daily copepod feeding activity was obtained by counting the number of algal cells left in the container with the two mature and healthy females, compared to the number of algae cells in the grazing control containers (no copepod) and corrected for algal growth (initial container counts).
Dead copepods were removed (1 dead female in C and LD and 2 in the HD) and replaced with new healthy females that had been kept in reserve containers (2 females per container) exposed to the same experimental conditions, including the daily transferring in new containers with fresh food suspensions. Grazing rates for dead copepods, were calculated assuming that they had lived for 1/2 day.
2.6. Survival
Female survival rate was monitored daily in all experimental incubations. In the “reproductive success” experiments (section 2.4) in June and July, 10 females per treatment were checked under the microscope to assure they were undamaged and healthy (actively swimming) before their daily transfer into new containers. In addition, in both June and July three replicates of two females per treatment were kept in 200 mL of food suspensions, and checked daily for swimming activity before being transferred to fresh seawater with new food. This second set of females was the one monitored for grazing activity in July (see section 2.5). Dead females, if any, were replaced with new healthy females (see section 2.5); thus survival rate in the 200 mL containers was calculated each day as the number of females still alive before the daily transfer to new food suspensions.
2.7. Statistical analysis
Statistical analyses were performed using the software Prism Graph Pad (v 6.0). Two-way analysis of variance (ANOVA) followed by Tukey test for post-hoc multiple comparisons (P<0.05) was performed to test for differences among treatments and over time for each measured fitness parameter using 10 replicates for egg production (see section 2. 6) and 6 replicates grazing (see section 2.5) experiments, respectively.
3. RESULTS
3.1. Alexandrium fundyense toxicity levels during the experiments
Toxin measurements for A. fundyense confirmed their neurotoxicity during both June and July experiments. Of the more than 20 naturally-occurring STX derivatives (Llewellyn, 2006), the A. fundyense culture contained nine derivatives represented by N-sulfocarbamoyl toxins (C1&2), gonyautoxins (GTX1&4, GTX2&3), decarbamoyl toxins (dcGTX3) neosaxitoxin (NEO) and saxitoxin (STX). The major toxins present (in order of relative abundance as molar % of total toxin) were N-sulfocarbamoyl toxins, followed by gonyautoxins, neosaxitoxin and saxitoxin (Figure 1A). The average toxin content (all derivatives) during the 7-day experiment was not significantly different between June and July (Student’s t-test; P = 0.36) (Figure 1B). During the June experiment, toxin content ranged between 0.01 and 0.03 ng STX equivalents cell−1, and there were no significant differences among experimental days. In July, toxin content was similar from Days 1 to 5 (mean: 0.014 ng STX equivalents cell−1) (Figure 1C). However, significantly higher toxin content was measured on Days 6 and 7 (Figure 1C). The higher toxicities corresponded to changes in NEO and STX derivatives, which showed a 2- and 10-fold increase, respectively, compared with previous days. The changes in the amount of NEO and STX also affected the relative proportion of the different toxins (% mol), as the proportion of NEO increased from 30% to 40% and STX from 1% to 10%, while N-sulfocarbamoyl and gonyautoxins toxins decreased to 27% and 23%, respectively.
Figure 1. Toxin profile of the dinoflagellate Alexandrium fundyense.
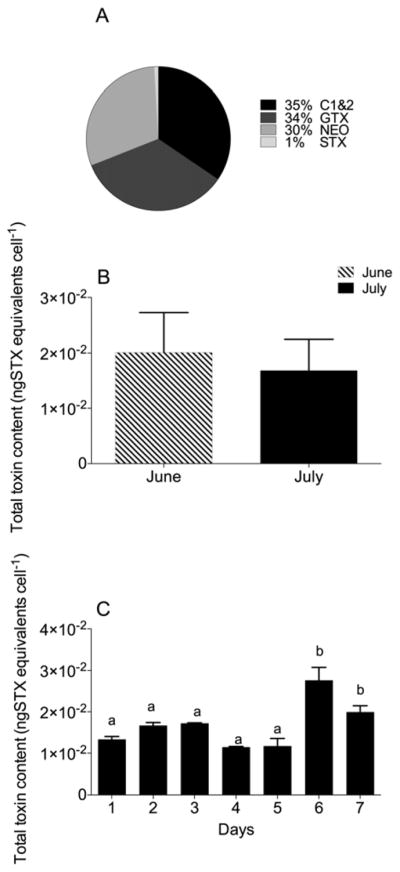
A) Percent molar PSP toxin composition for the July experiment. B) Average of total toxin content during 7-day experiments in June and July. C) Daily total toxin content measured during the July experiment; results of one-way ANOVA are reported on each bar: bars with the same letters are not significantly different.
C. finmarchicus females on the LD diet were exposed to a mean toxicity of 1.0 and 0.7 ng STX equivalents mL−1 in the June (Days 1–7) and July (Days 1–5) experiments, respectively. Mean toxicities for the HD treatment were 4-fold higher (4 and 2.8 ng STX equivalents mL−1 for June and July, respectively). The females were exposed to higher toxicities on Days 6 and 7 in the July experiment: 1.4 and 1 ng STX equivalents mL−1 for the LD; and 5.5 and 4 ng STX equivalents mL−1 for the HD treatment, respectively. Thus, the females experienced similar toxin profiles and toxin equivalency in the two experiments, with the exception of the last 2 experimental days in July.
3.2. Grazing activity
3.2.1. Ingestion rates
Calanus finmarchicus ingested A. fundyense in both LD and HD treatments over the 7-day experimental period with no significant differences compared with the control diet in terms of computed ingestion rates converted to μg C female−1 h−1 (Figure 2A). Ingestion rates averaged 1 μg C female−1 h−1 (±0.1SD) for control, LD and HD treatments (Figure 2A). These values are comparable to the mean ingestion rates reported for Calanus spp. adult females feeding on algae of similar cell size (Frost, 1972). Daily ingestion of toxins was computed from the toxicity measurements as STX equivalents per day (see section 3.1). STX ingestion for C. finmarchicus females feeding on the LD and HD did not change appreciably over time for the first 5 days of the experiment (Figure 2B). C. finmarchicus ingested on average 0.3 ng STX equivalents day−1 (±0.1SD) in the LD and 2 ng STX equivalents day−1 (±1SD) in the HD treatments between Day 1 and 5 (Figure 2B). On Days 6 and 7, cell toxicity levels were higher (Figure 2B) with corresponding higher STX ingestion rates in both LD and HD treatments (Figure 2B). Figure 2C shows daily carbon ingestion rates averaged across the 7-day experiment for each treatment. Ingestion of Rhodomonas sp. and A. fundyense in the LD treatment was proportional to their relative concentrations, showing no evidence of selective feeding under these experimental conditions.
Figure 2. Grazing activity for C. finmarchicus fed with LD and HD of A. fundyense and the control Rhodomonas spp. for 7 days.
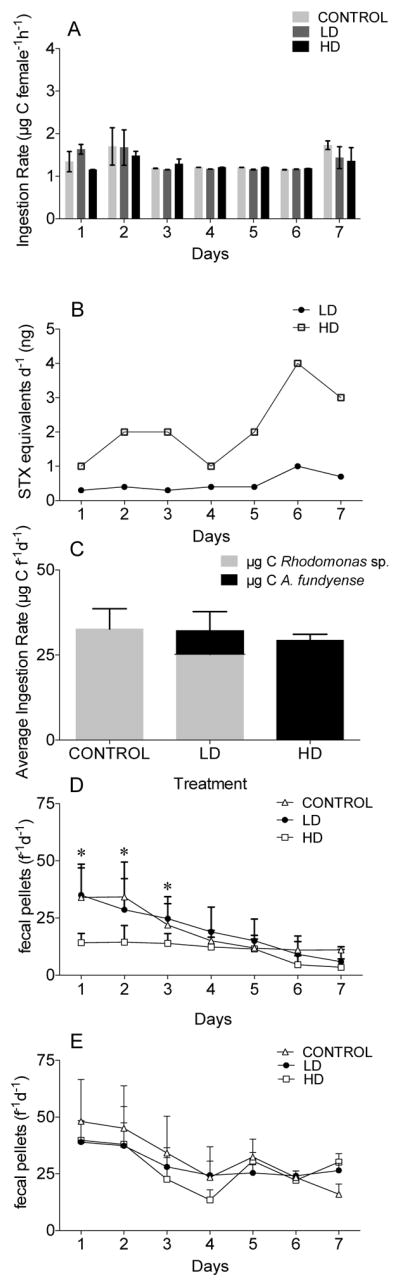
Results of grazing for July experiment with ingestion rates measured as carbon content (A), ingestion rates measured as STX equivalents (B) and average of daily ingestion rate (C). In D and E, fecal pellet production rate measured for females individually incubated in 100 mL containers (see text for details) in June (D) and July (E) experiments. * indicates significant differences between the treatments observed after statistical test (2-way ANOVA P<0.05).
3.2.2. Fecal pellet production
Fecal pellet production was similar in all three experimental treatments in June (Days 4–7; Figure 1D) and July (Days 1–7; Figure 2E). On Days 1–3, fecal pellet production was significantly lower in the HD treatment in the June experiment (Figure 2D). An overall decrease of the number of fecal pellets was observed over time in all treatments (Figure 2D, 2E); however, this decline was significant only for the control (both experiments) and LD treatment (June experiment; Table 3). Cumulative fecal pellet production confirmed the significant difference for females on the HD treatment in the June but not in the July experiment compared to the LD and the control diets.
Table 3.
Statistic results for Calanus finmarchicus fitness parameters significantly affected by treatment or time of exposure. For each parameter, a two way-ANOVA was performed using P<0.05 as significance cutoff.
| Factor | June | July | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | MS | F | p | df | MS | F | p | ||
| Fecal pellets | Treatment | 2 | 1807 | 25.16 | < 0.0001 | 2 | 247.6 | 2.53 | 0.0818 |
| Time | 6 | 1940 | 27.01 | < 0.0001 | 6 | 2136 | 21.89 | <0.0001 | |
| TxT | 12 | 187.9 | 2.61 | 0.0031 | 12 | 262.9 | 2.69 | 0.0022 | |
| Egg production daily | Treatment | 2 | 70.48 | 1.98 | 0.1409 | 2 | 168.7 | 1.70 | 0.1847 |
| Time | 6 | 211.4 | 5.94 | < 0.0001 | 6 | 3868 | 39.07 | < 0.0001 | |
| TxT | 12 | 36.18 | 1.01 | 0.4347 | 12 | 163.7 | 1.65 | 0.0801 | |
| Egg viability | Treatment | 2 | 9635 | 8.92 | 0.0003 | 2 | 45141 | 58.69 | < 0.0001 |
| Time | 6 | 4247 | 3.93 | 0.0015 | 6 | 13820 | 17.97 | < 0.0001 | |
| TxT | 12 | 2142 | 1.98 | 0.0336 | 12 | 602.8 | 0.78 | 0.6664 | |
Treatment: C (Rhodomonas sp.) LD and HD (A. fundyense); Time, 7-days; TxT: interaction between treatment and time; df, degree of freedom; MS, mean of squares; F, F ratio; P, probability. Statistically significant effect of factor is boldfaced.
3.3. Reproductive success
3.3.1. Egg production in the control
Daily egg production rates were highly variable over time with a decline observed in the controls in both experiments (Figure 3A, C). This decline was significant in the control and the experimental treatments (Figure 3A, C Table 3).
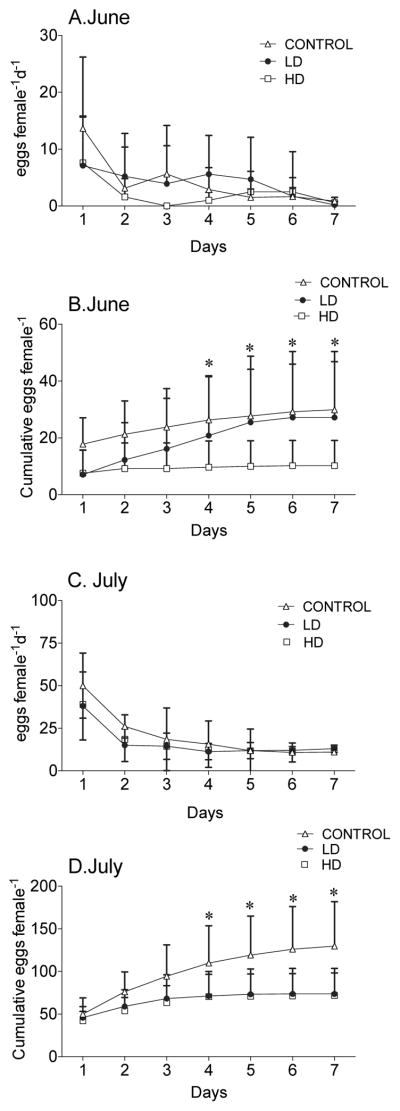
Figure 3. C. finmarchicus egg production rate after exposure to LD and HD of A. fundyense and the control Rhodomonas spp. for 7-days.
Daily (A) and cumulative (B) egg production rates over the 7-day experiment in June. In C) daily and cumulative (D) egg production rate over the 7-day experiment in July. * indicates significant differences between the treatments observed after statistical test (2-way ANOVA P<0.05).
3.3.2. Effect of A. fundyense on egg production
Alexandrium fundyense adversely affected C. finmarchicus egg production rates. Egg production rates were not significantly different between the three treatments on any specific day (Figures 3A, 3C), but the cumulative numbers of eggs produced were lower for females in the HD treatment for both June and July (Figures 3B, 3D, Table 3). In the June experiment, cumulative egg production per female on the HD diet was significantly lower starting on Day 4 and thereafter (Figure 3B). By Day 7, the cumulative number of eggs produced by females in the HD treatment averaged 16 (±2SD), and 29 and 28 (±4SD), in the control and LD treatments (Figure 3B). In July, cumulative egg production in both experimental treatments started to diverge from the control on Day 4 (Figure 3D). By the end of the experiments cumulative egg production for control, LD and HD females were 136, 74 and 72 total eggs female −1 respectively. Thus, females fed on A. fundyense diets had reduced fecundity of 35 to 47% in the HD treatment, while in the LD treatment reduced egg production was only observed in the July experiment.
3.3.2. Egg viability and naupliar production
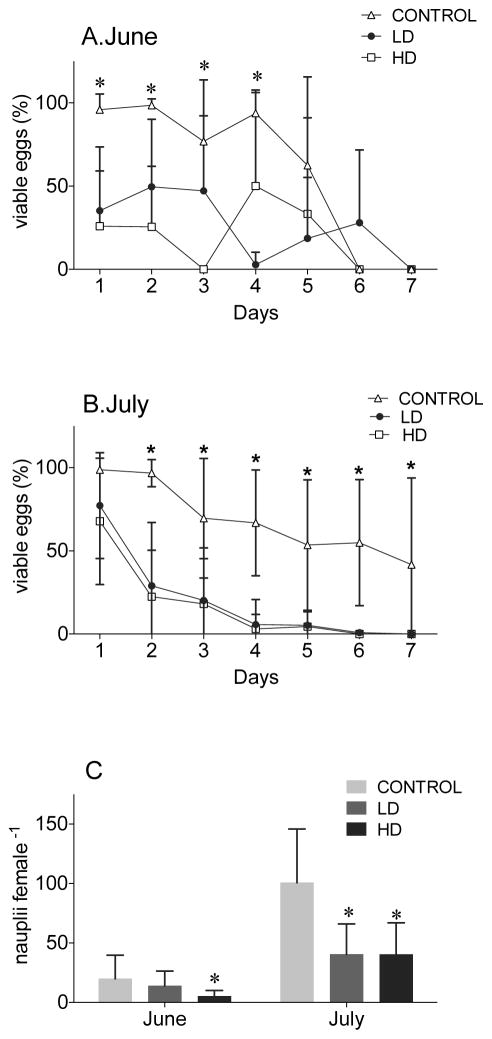
The negative effect of the A. fundyense diets was even more apparent in copepod egg hatching success. The number of viable eggs produced by females on both LD and HD diets of A. fundyense was reduced compared to eggs produced by females on the control diet in both experiments (Figure 4, Table 3). By Day 2, significantly lower hatching success was observed in both treatments (LD and HD) in both experiments (Figures 4A, 4B). In July, this significant difference between the control and the experimental diets persisted throughout the experiments (Figure 4B). In June, egg viability was more variable, and significant differences were only observed through Day 4 (Figure 4A). Thereafter, overall low egg production and viability in the June experiment led to no difference between the control and treatments (Figure 4A). The decline in egg viability over time was significant for all three treatments and in both experiments (Table 3).
Figure 4. Egg viability and effective recruitment (nauplii female−1) of C. finmarchicus adult females fed with LD and HD of A. fundyense and the control Rhodomonas spp. for 7 days.
Egg viability (%) for June (A) and July (B) experiments and effective recruitment (C) for both experiments. In C, the number of nauplii female−1 after 7 days exposure has been calculated for females that were still alive by Day 7. This corresponds to n=8 for C and LD and n=6 for HD in the June experiment and n=7 for C and LD and n=6 for HD in the July experiment. * indicates significant differences between the treatments observed after statistical test (2-way ANOVA P<0.05).
Effective recruitment was computed as the number of healthy nauplii produced during the 7-day experimental period by each female that survived the entire 7-day experiment. In both experiments a reduction was observed in the number of healthy nauplii produced by females fed with both the LD and HD diets compared to the control (Figure 4C). In June, this difference was only significant for the HD treatment while in July, the average number of nauplii per female was significantly lower in both LD and HD treatments (Figure 4C). In June on average, females in the LD and HD produced 65% and 25% of the number of healthy nauplii produced by control females (Figure 4C). In July, females feeding on LD and HD treatments produced on average 40% of the number produced by the control females (Figure 4C).
3.4. Survival
Calanus finmarchicus showed good survival rates during the two 7-day experiments in both LD and HD treatments of A. fundyense with no significant differences compared with the control diet (Figure 5). Similar survival rates were measured in two sets of conditions (100 mL and 200 mL containers) during the June and July experiments (Figure 5). In June, in the 200 mL containers (see section 2.6), survival rates on Day 7 were 100% for control and LD and 83% for the HD (1 dead female) (Figure 5A). In July, 100% of females fed the three treatments were still alive on Day 7 (Figure 5C). In the 100 mL flasks (see section 2.6), survival among treatments was similar but lower than in the 200 mL containers. In both June and July, survival rates on Day 7 ranged between 60% and 80% (Figure 5B, D). Overall the females were resistant to the toxic dinoflagellate with survival rates ranging between 60–100% over a long period (7 days) in all three treatments.
Figure 5. C. finmarchicus survival rates after exposure to LD and HD of A. fundyense and the control Rhodomonas spp. for 7 days.
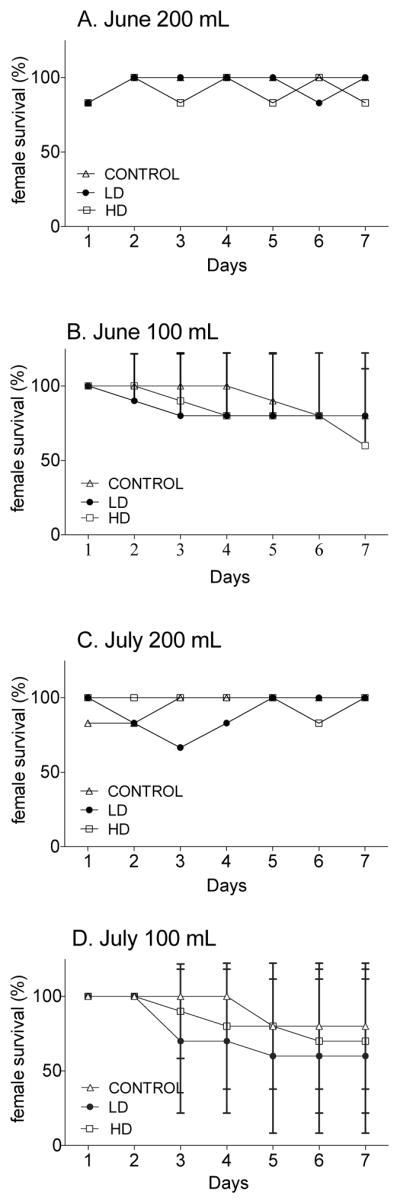
In A and B survival rates measured in June for females incubated in 100 mL (A) and 200 mL (B) containers. In C and D, survival rates measured in July for females incubated in 100 mL (C) and 200 mL (D) containers (see text for details).
4. Discussion
Diets of A. fundyense had little effect on copepod survival and grazing activity, however, there were effects on copepod reproduction. No difference in survival was found between the control and experimental treatments. Grazing activity was comparable between the control and the experimental treatments, and estimated daily carbon ingested was similar in all three treatments as well. However, overall egg production and egg viability were lower in the two experimental treatments, suggesting that feeding on the dinoflagellate reduced reproductive success, even at the lower dose.
The copepod C. finmarchicus co-occurs with A. fundyense blooms that are common in the waters of the Gulf of Maine (Petitpas et al., 2014). Alexandrium fundyense starts to appear in very low numbers during the annual spring bloom (Anderson, 1997), which is usually dominated by diatoms of the genera Thalassiosira, Chaetoceros and Skeletonema (103–105 cells mL−1) (Bigelow et al., 1926; Starr et al., 1999). During the summer, A. fundyense cell densities in the Gulf, including the Bay of Fundy, range between 1 to 55 cells mL−1 (Townsend et al. 2001), comparable to the LD treatment used in this study (50 cells mL−1). In contrast, the HD treatment was comparable to severe blooms > 100 cells mL−1 which have been occasionally reported in the Bay of Fundy (Anderson et al., 2014; McGillicuddy et al., 2014; Petitpas et al., 2014; Martin and White 1988). During bloom conditions, C. finmarchicus ingests A. fundyense (Turner and Borkman, 2005), even at modest bloom densities of 1 cell mL−1.
Resistance to toxic Alexandrium spp. in the form of high survival rates is widespread among copepods, including C. finmarchicus (Colin and Dam, 2002; Hassett, 2003; Liu and Wang, 2002; Teegarden, 1999; Teegarden and Cembella, 1996), although most of these studies were limited to 24 hours. The present study demonstrates that survival is not affected even when C. finmarchicus feeds exclusively on A. fundyense for a period of 7 days.
Exposure to A. fundyense diets did affect the reproductive success of Calanus finmarchicus. During the 7-day experimental period, egg production (total number of eggs) was significantly lower for adult females feeding on both experimental diets (July) and in the HD treatment (June) compared to the control.
Similar to our results with C. finmarchicus, Dutz (1998) reported a reduction in egg production in A. clausi females fed on a unialgal diet of the toxic A. lusitanicum for 6 days. A. clausi females feeding on the A. lusitanicum diet produced only 30% of the number of eggs produced by females in the control feeding on Rhodomonas baltica, which was a greater reduction in egg production than was observed in the present study for the HD diet. Both our study and that of Dutz (1998) suggest that copepods exposed to blooms of long duration (weeks to months) may show no increase in mortality due to the toxins, and would have an extended time period to accumulate toxins.
The decline of C. finmarchicus egg production over time in our experiments using the control diet has also been reported for other laboratory studies including those with similar experimental food levels (Hirche and Kwasniewski, 1997; Båmstedt et al., 1999; Niehoff et al., 2000; Jansen et al., 2006; Madsen et al., 2008). Thus, the decrease in egg production observed here for the control females feeding was expected. Moreover, the initial egg production rates (Day 1) in the June and July experiments were similar to the ones measured for C. finmarchicus collected in the western Atlantic during a similar period (June <10 eggs female−1 d−1; Plourde and Runge, 1993; July: 30–50 eggs female−1 d−1; Runge et al., 2006; Melle et al., 2014). Here, initial egg production in June averaged 13 eggs female−1 d−1 and 50 eggs female−1 d−1 in July. Furthermore, during the July experiment, the total number of eggs produced by the control females during the 7-day experiment (136 eggs female−1) (Figure 3D) was comparable to egg production rates reported in other studies for similar incubation periods (100–150 eggs female−1 – Hirche and Kwasniewski, 1997; Båmstedt et al., 1999).
The adverse effect of the A. fundyense diet was particularly evident when the number of hatched and healthy nauplii was considered as a measure of reproductive success. In July, on average, LD and HD females produced less than half of the number of viable eggs than in the control. In June, the average number of nauplii produced by the LD females was about 65% of that produced in the control, while the effect of the HD diet was even greater than in July, with only 25% viable eggs compared to the control diet. The effect of diet on egg viability in copepods has been studied both in the field and in laboratory experiments (Ianora and Miralto, 2010). Low egg viability has been reported for many different copepods, including Calanus spp. feeding on diatoms (Ceballos and Ianora, 2003; Ianora et al., 2003, 2004; Miralto et al., 1995, 1999; Poulet et al., 1995; Starr et al., 1999; Uye and Takamatsu, 1990). Total loss of egg viability has been reported in some copepods after 3 days on diets of the oxylipin-producing Skeletonema marinoi (Ceballos and Ianora, 2003). In contrast, egg viability in C. finmarchicus appears to be less affected by diatoms (Gerecht et al., 2013; Starr et al., 1999). Only a 20% reduction in egg viability was reported for C. finmarchicus feeding either exclusively on Thalassiorira nordenskioldii (104 cells mL−1) or on a mixture of diatoms (Thalassiorira nordenskioldii, Chaetoceros debilis, and Navicula sp.) even after 2 weeks exposure (Starr et al., 1999). A. fundyense had a much greater effect on egg viability in C. finmarchicus: hatching success declined to 50% and 30% after only 2 days even on the LD diet in June and July respectively. As expected, in the HD treatment the decline was even greater with hatching success of 25% (June) and 22% (July) compared to the control diet. These results suggest that A. fundyense blooms could have a significant impact on C. finmarchicus fitness even at A. fundyense densities that are common in the Gulf of Maine (Anderson, 1997; Anderson et al. 2005).
Whether the saxitoxins were responsible for the observed effects on reproductive success of C. finmarchicus remains unclear. The experiments reported here used Rhodomonas sp. as the control diet. Although it was confirmed that A. fundyense used in this study (strain GTC28) produced saxitoxins during the two 7-day experiments, there is the possibility that the negative effects were not caused by those toxins, but rather by other metabolites produced by A. fundyense. A dramatic reduction in egg production and hatching success in the copepod Temora stylifera fed on a non-toxic Alexandrium tamarense was attributed to other compounds that might be interfering with fertilization (Ianora et al., 2004). In addition, compounds that inhibit the growth of microalgae and heterotrophic protists have been identified in toxic and non-toxic strains of Alexandrium spp. (Tillmann and John, 2002; Tillmann et al., 2008). Thus, in the present study, it was difficult to determine whether the effects on reproductive success in C. finmarchicus were caused by the saxitoxins and/or by other compounds produced by the dinoflagellate. Alternatively, it has been suggested that toxic dinoflagellates such as Karenia brevis are nutritionally inadequate (Prince et al., 2006; Waggett et al., 2012). However, even though the fatty acid profile of A. fundyense was not measured in the present study, the fatty acid compositions of Alexandrium spp. are similar to many other dinoflagellate species that are considered to be high-quality food for copepods (Hammann et al., 2013; Ianora et al., 2004).
While survival rates were high, lower reproductive success was observed for both high (HD) and moderate (LD) levels of A. fundyense in the diet of C. finmarchicus females. This effect was only significant after several days of feeding on the experimental diets, and thus, could be missed in experiments of short duration (24–48 hrs). The results of this study suggest that blooms of A. fundyense in the Gulf of Maine may not affect C. finmarchicus survival, but they could lower the reproductive success of females maturing in June and July. In the summer, when high abundances of A. fundyense are common, particularly in the eastern Gulf of Maine, C. finmarchicus populations are dominated by the second generation (G1) (Miller et al., 1998). Starting in June, the population is represented by mid- and late-stage copepodites (CIV and CV) that are either preparing for diapause or maturing directly into adults and producing a third generation (G2) (Miller et al., 1998; Durbin et al., 2000; Fiksen, 2000). It is not clear whether A. fundyense might interfere with preparation for diapause in copepodites (stages CIV and CV). However, lower reproductive success by females encountering A. fundyense blooms could potentially reduce recruitment to the third generation (G2). A reduction in reproductive success might affect recruitment in the following winter/spring if the G2 contributes significantly to the winter adult population (e.g., Miller et al., 1998; Saumweber and Durbin, 2006).
In conclusion, the study provides evidence that the dinoflagellate A. fundyense is an environmental stressor for C. finmarchicus. Gene expression studies are consistent with A. fundyense being a stressor, as shown by intense transcriptional responses observed in C. finmarchicus females after a short- (2 days) and long-term (5 days) exposures (Roncalli, 2015). Upon ingestion of the dinoflagellate, the copepod experiences sub-optimal conditions in which physiological adjustments of its energy budget are required (Roncalli, 2015). The lower energy available to females feeding on the A. fundyense diets, which might be due to reduced food assimilation (Roncalli, 2015), may have contributed to the lower egg production and egg viability observed here.
Acknowledgments
We wish to extend our appreciation to the many colleagues who generously contributed to this study from the initial planning stages to its completion. We would like to thank the crew of the Elizabeth T. (Lobster Boat Cruises, MA), Adrianna Ianora, Adriana Zingone and Diana Sarno from the Stazione Zoologica ‘A. Dohrn’ (Naples), Daniel K. Hartline from the University of Hawaii at Manoa as well as Rajdeep Roy (ISRO-NRSC, Balanagar, Hyderabad, India) and Juliette Smith (Virginia Institute of Marine Science) for the toxin analyses. This research was supported by the National Science Foundation Grants OCE-1040597 and OCE-1235549 to Petra Lenz, the Cades Foundation of Honolulu and Mount Desert Island Biological Laboratory’s David W. Towle Fellowship 2012 to Vittoria Roncalli. Support for D. M. Kulis, D. M. Anderson, and J. T. Turner was provided through the Woods Hole Center for Oceans and Human Health, National Science Foundation Grant OCE-1314642 and National Institute of Environmental Health Sciences Grant 1-P01-ES021923-01. The views expressed herein are those of the authors and do not reflect the views of the funding agencies.
References
- Anderson DM. Bloom dynamics of toxic Alexandrium species in the northeastern US. Limnology and Oceanography. 1997;42(5 part2):1009–1022. [Google Scholar]
- Anderson DM, White AW. Marine biotoxins at the top of the food chain. Oceanus. 1992;35(3):55–61. [Google Scholar]
- Anderson D, Kulis D, Sullivan J, Hall S. Toxin composition variations in one isolate of the dinoflagellate Alexandrium fundyense. Toxicon. 1990;28:885–893. doi: 10.1016/0041-0101(90)90018-3. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Keafer BA, Kleindinst JL, McGillicuddy DJ, Martin JL, Norton K, Pilskaln CH, Smith JL, Sherwood CR, Butman B. Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms. Deep-Sea Research II. 2014;103:6–26. doi: 10.1016/j.dsr2.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Townsend DW, McGillicuddy DJ, Turner JT. The Ecology and Oceanography of Toxic Alexandrium fundyense Blooms in the Gulf of Maine. Deep-Sea Res II. 2005;52(19–21):2365–2876. [Google Scholar]
- Anderson DM, McGillicuddy DJ Jr, DeGrasse SL, Sellner KG, Bricelj VM, Turner JT, Townsend DW, Kleindinst JL, editors. Harmful Algae in the Gulf of Maine: Oceanography, Population Dynamics, and Toxin Transfer in the Food Web. Deep-Sea Res II. 2014;103:1–376. [Google Scholar]
- Båmstedt U, Nejstgaard JC, Solberg PT, Høisœter T. Utilisation of small-sized food algae by Calanus finmarchicus (Copepoda, Calanoida) and the significance of feeding history. Sarsia. 1999;84:19–38. [Google Scholar]
- Bigelow HB. Plankton of the offshore waters of the Gulf of Maine. Bulletin of the US Bureau of Fisheries. 1926;40:1–509. [Google Scholar]
- Bricelj VM, Shumway SE. Paralytic shellfish toxins in bivalve molluscs: occurrence, transfer kinetics and biotransformation. Reviews in Fisheries Science. 1998;6:315–383. [Google Scholar]
- Campbell RG, Teegarden GJ, Cembella AD, Durbin EG. Zooplankton grazing impacts on Alexandrium spp. in the nearshore environment of the Gulf of Maine. Deep-Sea Research II. 2005;52:2817–2833. [Google Scholar]
- Ceballos S, Ianora A. Different diatoms induce contrasting effects on the reproductive success of the copepod Temora stylifera. Journal of Experimental Marine Biology and Ecology. 2003;294:189–202. [Google Scholar]
- Colin SP, Dam HG. Latitudinal differentiation in the effects of the toxic dinoflagellate Alexandrium spp. on the feeding and reproduction of populations of the copepod Acartia hudsonica. Harmful Algae. 2002;1:113–125. [Google Scholar]
- Colin SP, Dam HG. Comparison of the functional and numerical responses of resistant versus non-resistant populations of the copepod Acartia hudsonica fed the toxic dinoflagellate Alexandrium tamarense. Harmful Algae. 2007;6:875–882. [Google Scholar]
- Conover R. Comparative life histories in the genera Calanus and Neocalanus in high latitudes of the northern hemisphere. Hydrobiologia. 1988;167:127–142. [Google Scholar]
- Darbyson E, Swain D, Chabot D, Castonguay M. Diel variation in feeding rate and prey composition of herring and mackerel in the southern Gulf of St Lawrence. Journal of Fish Biology. 2003;63:1235–1257. [Google Scholar]
- Davis CS. Zooplankton life cycles. In: Backus RH, editor. Georges Bank. MIT Press; Cambridge, MA: 1987. pp. 256–267. [Google Scholar]
- Deeds JR, Petitpas CM, Shue V, White KD, Keafer BA, McGillicuddy DJ, Milligan PJ, Anderson DM, Turner JT. PSP toxin levels and plankton community composition and abundance in size-fractionated vertical profiles during spring/summer blooms of the toxic dinoflagellate Alexandrium fundyense in the Gulf of Maine and on Georges Bank, 2007, 2008, and 2010: 1. Toxin levels. Deep-Sea Research II. 2014;103:329–349. doi: 10.1016/j.dsr2.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel S, Tande K. Does the spawning of Calanus finmarchicus in high latitudes follow a reproducible pattern? Marine Biology. 1992;113:21–31. [Google Scholar]
- Doucette GJ, Turner JT, Powell CL, Keafer BA, Anderson DM. Trophic accumulation of PSP toxins in zooplankton during Alexandrium fundyense blooms in Casco Bay, Gulf of Maine, April–June 1998. I. Toxin levels in A. fundyense and zooplankton size fractions. Deep-Sea Research II. 2005;52:2764–2783. [Google Scholar]
- Doucette G, Cembella A, Martin J, Michaud J, Cole T, Rolland R. Paralytic shellfish poisoning (PSP) toxins in North Atlantic right whales Eubalaena glacialis and their zooplankton prey in the Bay of Fundy, Canada. Marine Ecology Progress Series. 2006;306:303–313. [Google Scholar]
- Durbin E, Garrahan P, Casas M. Abundance and distribution of Calanus finmarchicus on the Georges Bank during 1995 and 1996. ICES Journal of Marine Science. 2000;57:1664–1685. [Google Scholar]
- Dutz J. Alexandrium lusitanicum: relationship between feeding and egg production. Marine Ecology Progress Series. 1998;175:97–107. [Google Scholar]
- Fields DM, Runge JA, Thompson C, Shema SD, Bjelland RM, Durif CMF, Skiftesvik AB, Browman HI. Infection of the planktonic copepod Calanus finmarchicus by the parasitic dinoflagellate, Blastodinium spp: effects on grazing, respiration, fecundity and fecal pellet production. Journal of Plankton Research. 2015;37:211–220. [Google Scholar]
- Fiksen Ø. The adaptive timing of diapause–a search for evolutionarily robust strategies in Calanus finmarchicus. ICES Journal of Marine Science. 2000;57:1825–1833. [Google Scholar]
- Frangópulos M, Guisande C, Maneiro I, Riveiro I, Franco J. Short-term and long-term effects of the toxic dinoflagellate Alexandrium minutum on the copepod Acartia clausi. Marine Ecology Progress Series. 2000;203:161–169. [Google Scholar]
- Frost B. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnology and Oceanography. 1972;17:805–815. [Google Scholar]
- Gerecht A, Carotenuto Y, Ianora A, Romano G, Fontana A, d’Ippolito G, Jakobsen HH, Nejstgaard JC. Oxylipin production during a mesocosm bloom of Skeletonema marinoi. Journal of Experimental Marine Biology and Ecology. 2013;446:159–165. [Google Scholar]
- Guillard R. Division rates. In: Stein JR, editor. Handbook of Phycological Methods. Cambridge University Press; Cambridge: 1973. pp. 289–312. [Google Scholar]
- Guisande C, Frangópulos M, Maneiro I, Vergara AR, Riveiro I. Ecological advantages of toxin production by the dinoflagellate Alexandrium minutum under phosphorus limitation. Maine Ecology Progress Seies. 2002;225:169–176. [Google Scholar]
- Hassett RP. Effect of toxins of the ‘red-tide’dinoflagellate Alexandrium spp. on the oxygen consumption of marine copepods. Journal of Plankton Research. 2003;25:185–192. [Google Scholar]
- Helland S, Nejstgaard J, Humlen R, Fyhn H, Båmstedt U. Effects of season and maternal food on Calanus finmarchicus reproduction, with emphasis on free amino acids. Marine Biology. 2003;142:1141–1151. [Google Scholar]
- Hirche HJ. Egg production of Calanus finmarchicus at low temperature. Marine Biology. 1990;106:53–58. [Google Scholar]
- Hirche HJ, Kwasniewski S. Distribution, reproduction and development of Calanus species in the Northeast Water in relation to environmental conditions. Journal of Marine Systems. 1997;10:299–317. [Google Scholar]
- Ianora A, Miralto A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: a review. Ecotoxicology. 2010;19:493–511. doi: 10.1007/s10646-009-0434-y. [DOI] [PubMed] [Google Scholar]
- Ianora A, Poulet SA, Miralto A. The effects of diatoms on copepod reproduction: a review. Phycologia. 2003;42:351–363. [Google Scholar]
- Ianora A, Turner JT, Esposito F, Carotenuto Y, d’Ippolito G, Romano G, Fontana A, Guisande C, Miralto A. Copepod egg production and hatching success is reduced by maternal diets of a non-neurotoxic strain of the dinoflagellate Alexandrium tamarense. Marine Ecology Progress Series. 2004;280:199–210. [Google Scholar]
- Ianora A, Miralto A, Poulet SA, Carotenuto Y, Buttino I, Romano G, Casotti R, Pohnert G, Wichard T, Colucci-D’Amato L. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature. 2004;429:403–407. doi: 10.1038/nature02526. [DOI] [PubMed] [Google Scholar]
- Jansen S, Riser CW, Wassmann P, Bathmann U. Copepod feeding behaviour and egg production during a dinoflagellate bloom in the North Sea. Harmful Algae. 2006;5:102–112. [Google Scholar]
- Liu S, Wang WX. Feeding and reproductive responses of marine copepods in South China Sea to toxic and nontoxic phytoplankton. Maine Biology. 2002;140:595–603. [Google Scholar]
- Llewellyn LE. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Natural Products Reports. 2006;23:200–222. doi: 10.1039/b501296c. [DOI] [PubMed] [Google Scholar]
- Madsen SJ, Nielsen TG, Tervo OM, Soderkvist J. Importance of feeding for egg production in Calanus finmarchicus and Calanus glacialis during the Arctic spring. Marine Ecology Progress Series. 2008;353:177–190. [Google Scholar]
- Martin JL, White AW. Distribution and abundance of the toxic dinoflagellate Gonyaulax excavata in the Bay of Fundy. Canadian Journal of Fisheries and Aquatic Sciences. 1988;45:1968–1975. [Google Scholar]
- McGillicuddy DJ, Townsend DW, Keafer BA, Thomas M, Anderson DM. Georges Bank: a leaky incubator of Alexandrium fundyense blooms. Deep-Sea Research II. 2014;103:163–173. doi: 10.1016/j.dsr2.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meise C, O’Reilly J. Spatial and seasonal patterns in abundance and age-composition of Calanus finmarchicus in the Gulf of Maine and on Georges Bank: 1977–1987. Deep-Sea Research II. 1996;43(7):1473–1501. [Google Scholar]
- Melle W, Runge J, Head E, Plourde S, Castellani C, Licandro P, Pierson J, Jonasdottir S, Johnson C, Broms C. The North Atlantic Ocean as habitat for Calanus finmarchicus: environmental factors and life history traits. Progress in Oceanography. 2014;129:244–284. [Google Scholar]
- Menden-Deuer S, Lessard EJ. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnology and Oceanography. 2000;45:569–579. [Google Scholar]
- Miller CB, Lynch DR, Carlotti F, Gentleman W, Lewis CV. Coupling of an individual-based population dynamic model of Calanus finmarchicus to a circulation model for the Georges Bank region. Fisheries Oceanography. 1998;7:219–234. [Google Scholar]
- Miralto A, Ianora A, Poulet S. Food type induces different reproductive responses in the copepod Centropages typicus. Journal of Plankton Research. 1995;17:1521–1534. [Google Scholar]
- Miralto A, Barone G, Romano G, Poulet S, Ianora A, Russo G, Buttino I, Mazzarella G, Laabir M, Cabrini M. The insidious effect of diatoms on copepod reproduction. Nature. 1999;402:173–176. [Google Scholar]
- Niehoff B, Hirche HJ, Båmstedt U. The reproduction of Calanus finmarchicus in the Norwegian Sea in spring. Sarsia. 2000;85:15–22. [Google Scholar]
- Oshima Y. Postcolumn derivatization liquid chromatography method for paralytic shellfish toxins. J AOAC Int. 1995;78:528–532. [Google Scholar]
- Petitpas CM, Turner JT, Deeds JR, Keafer BA, McGillicuddy DJ, Jr, Milligan PJ, Shue V, White KD, Anderson DM. PSP toxin levels and plankton community composition and abundance in size-fractionated vertical profiles during spring/summer blooms of the toxic dinoflagellate Alexandrium fundyense in the Gulf of Maine and on Georges Bank, 2007, 2008, and 2010: 2. Plankton community composition and abundance. Deep-Res Research II. 2014;103:350–367. doi: 10.1016/j.dsr2.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planque B, Hays GC, Ibanez F, Gamble JC. Large scale spatial variations in the seasonal abundance of Calanus finmarchicus. Deep-Sea Research I. 1997;44:315–326. [Google Scholar]
- Plourde S, Runge JA. Reproduction of the planktonic copepod Calanus finmarchicus in the Lower St. Lawrence Estuary: relation to the cycle of phytoplankton production and evidence for a Calanus pump. Marine Ecology Progress Series. 1993;102:217–227. [Google Scholar]
- Poulet S, Ianora A, Laabir M, Breteler WK. Towards the measurement of secondary production and recruitment in copepods. ICES Journal of Maine 1995 [Google Scholar]
- Prince EK, Lettieri L, McCurdy KJ, Kubanek J. Fitness consequences for copepods feeding on a red tide dinoflagellate: deciphering the effects of nutritional value, toxicity, and feeding behavior. Oecologia. 2006;147:479–488. doi: 10.1007/s00442-005-0274-2. [DOI] [PubMed] [Google Scholar]
- Roncalli V. PhD disseretation. Universikty of Hawaii; 2015. The effect of the toxic dinoflagellate Alexandrium fundyense on the calanoid copepod Calanus finmarchicus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge J, Plourde S, Joly P, Niehoff B, Durbin E. Characteristics of egg production of the planktonic copepod, Calanus finmarchicus, on Georges Bank: 1994–1999. Deep-Sea Research II. 2006;53:2618–2631. [Google Scholar]
- Saumweber WJ, Durbin EG. Estimating potential diapause duration in Calanus finmarchicus. Deep-Sea Research II. 2006;53:2597–2617. [Google Scholar]
- Sherman K, Smith WG, Green JR, Cohen EB, Berman MS, Marti KA, Goulet JR. Zooplankton production and the fisheries of the northeastern shelf. In: Backus RH, editor. Georges Bank. MIT Press; Cambridge, MA: 1987. pp. 268–282. [Google Scholar]
- Shumway SE. A review of the effects of algal blooms on shellfish and aquaculture. Journal of the World Aquaculture Society. 1990;21:65–104. [Google Scholar]
- Shumway SE, Sherman-Caswell S, Hurst J. Paralytic shellfish poisoning in Maine: monitoring a monster. Journal of Shellfish Research. 1988;7:643–652. [Google Scholar]
- Seixas P, Coutinho P, Ferreira M, Otero A. Nutritional value of the cryptophyte Rhodomonas lens for Artemia sp. Journal of Experimental Marine Biology and Ecology. 2009;381(1):1–9. [Google Scholar]
- Sopanen S, Setälä O, Piiparinen j, Erler K, Kremp A. The toxic dinoflagellate Alexandrium ostenfeldii promotes incapacitation of the calanoid copepods Eurytemora affinis and Acartia bifilosa from the northern Baltic Sea. Journal of Plankton Research. 2011;33:1564–1573. [Google Scholar]
- Starr M, Runge JA, Therriault JC. Effects of diatom diets on the reproduction of the planktonic copepod Calanus finmarchicus. Sarsia. 1999;84:379–389. [Google Scholar]
- Sun J, Liu D. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research. 2003;25:1331–1346. [Google Scholar]
- Teegarden GJ. Copepod grazing selection and particle discrimination on the basis of PSP toxin content. Marine Ecology Progress Series. 1999;181:163–176. [Google Scholar]
- Teegarden GJ, Cembella AD. Grazing of toxic dinoflagellates, Alexandrium spp., by adult copepods of coastal Maine: implications for the fate of paralytic shellfish toxins in marine food webs. Journal of Experimental Marine Biology and Ecology. 1996;196:145–176. [Google Scholar]
- Teegarden GJ, Campbell RG, Durbin EG. Zooplankton feeding behavior and particle selection in natural plankton assemblages containing toxic Alexandrium spp. Marine Ecology Progress Series. 2001;218:213–226. [Google Scholar]
- Teegarden GJ, Cembella AD, Capuano CL, Barron SH, Durbin EG. Phycotoxin accumulation in zooplankton feeding on Alexandrium fundyense-vector or sink? Journal of Plankton Research. 2003;25:429–443. [Google Scholar]
- Teegarden GJ, Campbell RG, Anson DT, Ouellett A, Westman BA, Durbin EG. Copepod feeding response to varying Alexandrium spp. cellular toxicity and cell concentration among natural plankton samples. Harmful Algae. 2008;7:33–44. [Google Scholar]
- Tillmann U, John U. Toxic effects of Alexandrium spp. on heterotrophic dinoflagellates: an allelochemical defence mechanism independent of PSP-toxin content. Marine Ecology Progress Series. 2002;230:47–58. [Google Scholar]
- Tillmann U, Alpermann T, John U, Cembella A. Allelochemical interactions and short-term effects of the dinoflagellate Alexandrium on selected photoautotrophic and heterotrophic protists. Harmful Algae. 2008;7:52–64. [Google Scholar]
- Townsend DW, Pettigrew NR, Thomas AC. Offshore blooms of the red tide dinoflagellate, Alexandrium sp., in the Gulf of Maine. Cont Shelf Res. 2001;21:347–369. [Google Scholar]
- Turner JT. Harmful algae interactions with marine planktonic grazers. In: Granéli E, Turner JT, editors. Ecology of harmful algae. Springer-Verlag; 2006. pp. 259–270. [Google Scholar]
- Turner JT. Zooplankton community grazing impact on a bloom of Alexandrium fundyense in the Gulf of Maine. Harmful Algae. 2010;9:578–589. doi: 10.1016/j.hal.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JT. Planktonic marine copepods and harmful algae. Harmful Algae. 2014;32:81–93. [Google Scholar]
- Turner JT, Borkman DG. Impact of zooplankton grazing on Alexandrium blooms in the offshore Gulf of Maine. Deep-Sea Reearch II. 2005;52:2801–2816. [Google Scholar]
- Turner JT, Tester PA. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnology and Oceanography. 1997;42(5 part 2):1203–1213. [Google Scholar]
- Turner JT, Doucette GJ, Keafer BA, Anderson DM. Trophic accumulation of PSP toxins in zooplanbkton during Alexandrium fundyense blooms in Casco Bay, Gulf of Maine, April-June 1998. II. Zooplankton abundance and size-fractionated community composition. Deep-Sea Research II. 2005;52:2784–2800. [Google Scholar]
- Uye S, Takamatsu K. Feeding interactions between planktonic copepods and red-tide flagellates from Japanese coastal waters. Marine Ecology Progress Series. 1990;59:97–107. [Google Scholar]
- Waggett RJ, Hardison DR, Tester PA. Toxicity and nutritional inadequacy of Karenia brevis: synergistic mechanisms disrupt top-down grazer control. Mar Ecol Prog Ser. 2012;444:15–30. [Google Scholar]