Abstract
Although previously proposed that chronic scleroderma should be cared for clinically and early rehabilitation should be performed in hospital by a chest physical therapist, little evidence is currently available on its benefits. Therefore, this study demonstrated the benefits of short-term pulmonary rehabilitation during hospitalization in a female patient with chronic scleroderma. The aim of rehabilitation was to improve ventilation and gas exchange by using airway clearance, chest mobilization, and breathing-relearning techniques, including strengthening the respiratory system and the muscles of the limbs by using the BreathMax® device and elastic bands. Gross motor function and activities of daily life were regained by balancing, sitting, and standing practices. Data on minimal chest expansion, high dyspnea, high respiratory rate, and low maximal inspiratory mouth pressure were recorded seven days before rehabilitation or at the baseline period. But there was a clinically significant improvement in dyspnea, chest expansion, maximal inspiratory mouth pressure, and respiratory rate, when compared to baseline data, which were recorded by a chest physical therapist during seven days of rehabilitation. Furthermore, physicians decided to stop using a mechanical ventilator, and improvement in functional capacity was noted. Therefore, in the case of chronic and stable scleroderma, short-term rehabilitation during hospitalization for chest physical therapy possibly shows clinical benefits by improving both pulmonary function and physical performance.
Keywords: chest physical therapy, rehabilitation, scleroderma, single case research design
Introduction
Scleroderma is a chronic multisystem disease, and it is randomly present in population worldwide.1 It mainly affects the microvascular system and connective tissue, causing alternations in the skin (scleroderma) and the internal organs2 such as the heart, gastrointestinal tract, kidneys, and lungs, with fibrotic and vascular deposition.3 The clinical features of scleroderma include significant changes in the skin such as hyperpigmentation or hypopigmentation, similar to Raynaud’s phenomenon and hard end-feel, as well as multiple digital ulcers, shortened tips of all fingers, keratosis, and abnormalities in peripheral circulation and sensation.4 Evidence from the previous study on distinguishable complications in 117 patients with systemic sclerosis showed bacterial aspiration, pneumonia, and opportunistic infection in various organs.5 Despite poor prognosis, early diagnosis with aggressive treatment will possibly increase survival rate. Major problems arise due to skin contracture or muscle shortening in all extremities and the thoracic chest wall. Therefore, progressive generalized scleroderma induces shortness of breath, ineffective coughing, and respiratory failure6 because of respiratory muscle weakness and subcutaneous chest wall stiffness.2,7 Incidentally, the main causes of death are upper airway obstruction with excessive secretion,8,9 pulmonary hypertension, hypoxemia, and acute respiratory failure.10,11 In addition, complicated pulmonary disorder affects exercise capacity12 and physical function directly because of low aerobic capacity and musculoskeletal disorder.13 In the case of scleroderma, admittance into the intensive care unit (ICU) and the prolonged use of a mechanical ventilator, due to respiratory failure, cause significant weakness of the respiratory muscles not only due to pathology but also due to prolonged bed rest in the ICU. Previous evidence has proved that prolonged stay in an ICU (7.7 ± 3.7 days) and need for a mechanical ventilator (4.6 ± 2.5 days) result in a decrease in respiratory muscle strength by approximately 12%.14 Retained secretion in airways is mostly common in patients who are critically ill and those on mechanical ventilation because of an imbalance between overproductive mucus and impaired clearance capacities.15 Therefore, it can be concluded from the aforementioned overview that many problems in patients depending on a mechanical ventilator in the ICU are due to shortness of breath, dyspnea, retained secretion, respiratory muscle weakness, insufficient secretion clearance, inability to wean from a mechanical ventilator due to ventilation–oxygenation impairment, general muscle weakness, and loss of activities of daily life (ADLs). Therefore, short-term pulmonary rehabilitation is very important for regaining overall good health, during either an acute phase in the ICU or a recovery period before being discharged.16 The goals of the chest physical therapist regarding rehabilitation have been proposed and standardized by the cardiovascular and essential pulmonary guidelines of 2001 from the American Physical Therapy Association.17 Improvement of ventilation via gas exchange; reduction of effort and control in breathing; improvement in coughing patterns, including increased respiratory rate and limb muscle strength; and improvement of functional capacity in terms of both gross motor function and ADL related to quality of life are considered as final outcomes before being discharged.17 In addition, a previous report suggested that the role of physical therapy (PT) and coming off a mechanical ventilator, during the critical period of recovering physical and respiratory functions, prevent the effects of bed rest and improve health status. Integrated programs that deal with both whole-body PT and pulmonary care are needed to manage these patients, but scientific evidence offers limited support for them.18 Previous evidence showed that the general hospitalized program suggests various techniques such as postural drainage (PD), vibrations, and deep breathing exercises with incentive spirometry and an oscillating positive expiratory pressure device to remove secretion.19 The effects of previously positive endotracheal suction, mobilization, and adjusted position by a chest physical therapist, who visited 146 ICU patients 24 hours daily, reduced the time on a mechanical ventilator and prevented recurrent respiratory infection.16 Moreover, a report by Clini and Ambrosino20 summarized that most of the interventions applied such as mobilization (posture, passive/active limb exercise, and continuous rotational therapy), manual therapy (manual hyperinflation, percussion, and vibration), and muscle exercise training (respiratory and peripheral muscles) should be performed in the ICU, but their research lacked the study on long-term outcomes. This study treated a female patient with postrespiratory failure due to severe scleroderma, and physicians decided to stop using mechanical ventilation to prevent pulmonary complications and prepare the patient for discharge with the least chance of chronic scleroderma. These results showed the benefits of short-term rehabilitation by a visiting chest physical therapist, which can be possibly applied to future scleroderma cases.
Materials and Methods
Experimental design and case characteristics
The data on all parameters at the baseline period or seven days before and during seven days of short-term rehabilitation by a chest physical therapist were collected and presented with a single-case research design and an A–B pattern, or comparison between observation (A) and intervention (B) periods. All data from this study were agreed upon, as informed consent was obtained from the patient during rehabilitation and before any publication. Seven points of mean, either at baseline or during rehabilitation periods of chest physical therapy (CPT), were plotted on visual line graphs, as well as a mean line. The trend line from baseline or the observation period was presented on graphs. Autocorrelations of each period were also calculated and shown on a graph. Finally, indication of clinically significant improvement during seven days of rehabilitation was analyzed, with the trend line compared to the baseline by using the protocol of a single-case study research design model.21
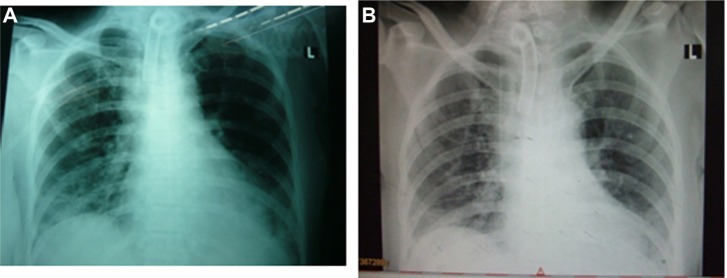
An alert 45-year-old Thai female patient was admitted to Maharaj Nakorn Chiang Mai Hospital, Chiang Mai University, Thailand, and diagnosed with diffused scleroderma with respiratory failure. She was referred ethically to a chest physical therapist by a medical physician, due to her inability to stop using a mechanical ventilator and her total dependence on full support during physical ambulation within the ward. History of the patient showed impairment in terms of inactive neurological problems of right thalamic hemorrhage accompanied by left spastic hemiparesis and hypertension with hypertensive retinopathy since 2006, but all of these problems had been resolved or stabilized. A basic examination by a physician showed abnormalities on the skin, including thickening on the face, chest wall, and elbow regions. An echocardiogram (ECHO) showed normal cardiovascular function, no murmur, and regular pulse, but there was mild atrial fibrillation and pulmonary hypertension with 50% of ejection fraction. Due to previous respiratory failure and unsuccessful spontaneous breath for three weeks in the ICU, support was necessary from a mechanical ventilator via tracheostomy tube under controlled mandatory ventilation (CMV) mode [TV = 3.5 mL, Ti = 0.2, FiO2 = 0.45, respiratory rate (RR) = 18 bpm, positive end expiratory pressure (PEEP) = 5 cmH2O], alternated with a continuous positive airway pressure (CPAP) mode (PS = 25 cmH2O, FiO2 = 0.45). Previous results of a three-day arterial blood gas (ABG) investigation showed impairment with hypoventilation (PaCO2 = 52.0 ± 1.4 mmHg), mild hypoxemia (PaO2 = 75.2 ± 2.2 mmHg), and slight respiratory acidosis (pH = 7.30 ± 2.5). Moreover, other laboratory tests showed anemia (Hb = 8.5 g/dL and Hct = 28.5%) and low protein (total protein = 4.9 g/dL and albumin = 2.0 g/dL), whereas no microbial growth was seen in the sputum, with liver function being normal (Table 1). Chest radiography (CXR) prior to the rehabilitation program (Fig. 1A) showed infiltration with secretion in the right and left lower lungs. Routine drugs were administered continuously with mucolytic (Fluimucil® A600, Zambon), antidepressant (Fluoxetine 20 mg, Mylan Pharmaceuticals Inc), and histamine-2 blocker (Ranitidine, Cadila Healthcare Limited) three weeks before the CPT program started.
Table 1.
Laboratory results of the patient at baseline and after rehabilitation.
| PARAMETERS (REFERENCE RANGE) | BASELINE (DAY 1–7) | REHABILITATION (DAY 8–14) |
|---|---|---|
| Arterial blood gases (ABG) | ||
| pH (7.35–7.45) | 7.30 ± 2.5 | 7.45 ± 3.4 |
| PaCO2 (mmHg) (35–45) | 52.0 ± 1.4 | 40.0 ± 2.3 |
| PaO2 (mmHg) (>80) | 75 ± 2.2 | 85 ± 3.0 |
| HCO3− (mEq/L) (22–26) | 24.5 ± 1.8 | 25.3 ± 1.2 |
| O2 Sat (%) (>95) | 95 ± 2% | 98 ± 2% |
|
| ||
| Ventilator setting | CMV CPAP | T-piece |
| TV = 350 mL Ps = 25 cmH2O | O2 = 4 Lpm | |
| Ti = 0.2 | ||
| FiO2 = 0.45 | ||
| RR = 18 bpm | ||
| PEEP = 5 | ||
|
| ||
| Hematology (reference range) | (Day 1) | (Day 14) |
| Hb (g/dL) (10–16) | 8.5 | 12.6 |
| Hct (%) (36–50) | 28.5 | 35.4 |
| WBC (103/μL) (5–10) | 14,836 | 16,700 |
| Neutrophil (%) (40–75) | 72.0 | 78.3 |
| Eosinophil (%) (1–3) | 1.0 | 0.9 |
| Basophil (%) (0–1) | 1.0 | 0.5 |
| Lymphocyte (%) (25–35) | 15.0 | 16.5 |
| Monocyte (%) (2–10) | 11.0 | 7.5 |
| Platelet (103/μL) (140–440) | 61,900 | 541,000 |
|
| ||
| Blood chemistry (reference range) | (Day 1) | (Day 14) |
| Glucose (mg/dL) (65–100) | 112 | 109 |
| B.U.N. (mg/dL) (0–20) | 12 | 10 |
| Creatinine (mg/dL) (0.6–1.2) | 1.1 | 1.2 |
| Na (mEq/L) (135–145) | 139 | 124 |
| K (mEq/L) (3.8–4.8) | 4.7 | 3.6 |
| Cl (mEq/L) (95–105) | 109 | 89 |
| Total CO2 (mmol/L) (19–24) | 23 | 32 |
|
| ||
| Liver function test (reference range) | (Day 1) | (Day 14) |
| Total protein (g/dL) (6.3–7.9) | 4.9 | 6.3 |
| Albumin (g/dL) (3.5–5.0) | 2.0 | 2.6 |
| Globulin (g/dL) (2.0–3.5) | 2.5 | 2.2 |
| Alk. Phos. (U/L) (40–100) | 57.1 | 57 |
| Cholesterol (mg/dL) (<200) | 147 | 99 |
| AST (U/L) (10–42) | 27 | 18 |
| ALT (U/L) (10–40) | 45 | 13 |
| Total bilirubin (mg/dL) (0.2–1.0) | 0.5 | 0.24 |
| Direct bilirubin (mg/dL) (0.1–0.2) | 0.22 | 0.12 |
|
| ||
| Sputum culture | No bacterial growth | No bacterial growth |
Figure 1.
Chest X-rays from the A–P position: (A) pretreatment (Day 1) and (B) final day of rehabilitation (Day 14).
Rehabilitation program and assessment
The CPT program carried out rehabilitation for approximately 1–2 hours per visit at 9 a.m. and 2 p.m. All parameters were assessed during a 7-day baseline period, such as chest wall expansion at the middle lobe (at the nipple level) by chest caliper (Martin Breadth Caliper), dyspnea score by visual chart (on a 0–10 scale; Borg scale), and maximal inspiratory mouth pressure (PImax) by an NIF kit™ (Smiths Medical Asd, Inc). Furthermore, through lung auscultation, breath sound and air entry into the lower lungs were found to be decreased, including fine crepitation or crackle on both lower lungs, especially on the right side. Musculoskeletal evaluation showed moderate muscle power of grade 3 in all extremities with regard to the range of motion in both shoulder joints at flexion and abduction and trunk lateral flexion and rotation while the patient suffered from scleroderma. Finally, bed mobility and gross motor function were evaluated by bed transfer, lying to sitting and sitting to standing positions, and walking while depending on full support.
Goals of rehabilitation
All rehabilitation programs were performed by an expert physical therapist, who had more than 15 years of experience in cardiopulmonary rehabilitation in a hospital, and a legal PT license was approved by the Physical Therapy Council of Thailand.
The short-term goals for CPT were improvement of (1) ventilation and gas exchange, (2) chest mobility and breathing work, (3) strength of respiratory muscles and limbs, and (4) functional ability and gross motor function within one week. The desired outcomes of rehabilitation were an end to the use of a mechanical ventilator and independence in carrying out ADL.22 The treatment program was carried out according to the goals and problems list, ie, improvement of pulmonary ventilation and gas exchange performed by modified PD positions for 10–15 minutes, percussion, and suction until both lower basal lungs were cleared of secretion. Modified PD positions were set because the CXR film presented infiltration in both basal lungs, and the positions consisted of head bent down by 40°, lying sideways on the midline, and leaning forward or backward.23 Synchronized manual percussion and interval vibration were performed for 5–10 minutes per segmental position, with lower costal breathing, huffing, or coughing being practiced in a serial manner that was essentially similar to the active cycle of breathing techniques.24 Suction was carried out in the tracheostomy tube to clear secretion. The total time for clearing the airway was approximately 20–30 minutes. The procedure for improving chest wall flexibility and breathing work was performed using chest mobilization techniques that comprised trunk rotation, lateral flexion, flexion and extension, and sternum flexibility in the sitting position25 for 10 repeated turns of each pattern. After that, lower costal breathing and the diaphragmatic breathing were stimulated by exercise for approximately 10–20 minutes to improve the breathing pattern.
Improving the strength of the respiratory muscle was performed using a BreathMax® device.26 With the PImax score being 3.5 cmH2O before intervention, the resistant threshold was set by dipping the end of the BreathMax® device tube underwater at 2.0, 2.0, 2.5, 2.5, 3.0, 3.0, and 3.5 cmH2O consecutively. The BreathMax® device controlled the bubble sound slowly during inspiration, preferably performed 10 times, depending on when a comfortable RR was attained. Strengthening the respiratory muscle was enhanced by upper limb exercises using yellow elastic bands (Smart Med®) in a sitting position on a bed. Each pattern was performed repeatedly 10 times, with two sessions in the morning and two in the evening. However, the lower limb gained the desired strength by using the elastic bands in a sitting position. Finally, the improvement of functional ability and gross function was achieved by carrying out strengthening exercises on the lower limbs and training for balance during sitting, standing, and walking with a rolling walker.27 There was the utmost concern for overloading work while performing all the treatments. If the level of SatO2 percentage dropped to less than 95 and dyspnea score from 10 to 5 and the hemodynamic demonstrated significant changes28 such as more tachypnea and accessory muscle use, decreased heart rate or blood pressure, or change in consciousness, O2 supplementation via the tracheostomy tube was conducted at 5 Lpm, using an inflated manual resuscitating bag.
Clinically significant analysis
This study was a single-case research design model; therefore, the mean of each parameter such as that of dyspnea, RR, chest expansion, PImax, and trend line in the baseline period was drawn on a graph. The clinical benefits of rehabilitation were analyzed statistically by using the Bloom table.
Results
Before rehabilitation using sternocleidomastoid on a mechanical ventilator, the patient in this study was found to have dominant symptoms of shortness of breath or severe dyspnea (a mean of 5.85 ± 0.69; Fig. 2) and tachypnea (RR at 30 ± 1.68 bpm; Fig. 3), as well as minimal chest expansion (0.93 ± 0.34 cm; Fig. 4) and respiratory muscle strength (PImax = 3.14 ± 0.69 cmH2O; Fig. 5).
Figure 2.
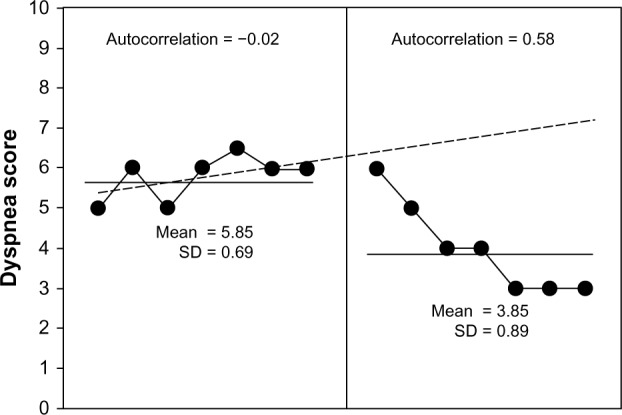
Dyspnea score after seven days at baseline (left) and during seven days of rehabilitation (right). It was evident from the Bloom table that all of the points during rehabilitation by CPT were under the trend line from the baseline period, thus indicating a clinically significant change.
Figure 3.
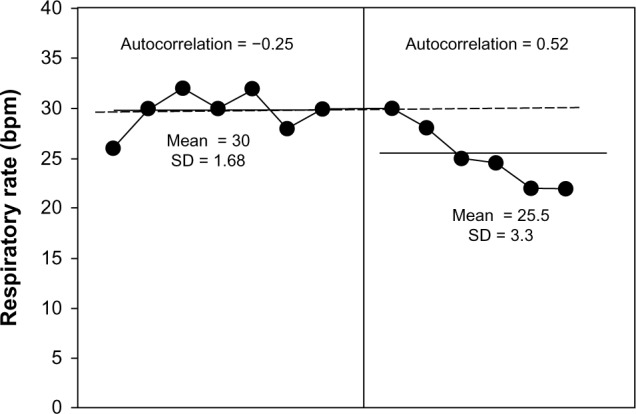
RR after seven days at baseline (left) and during seven days of rehabilitation by CPT (right). It was evident from the Bloom table that six points from all of those during rehabilitation by CPT were under the trend line from the baseline period, thus indicating a clinically significant change.
Figure 4.
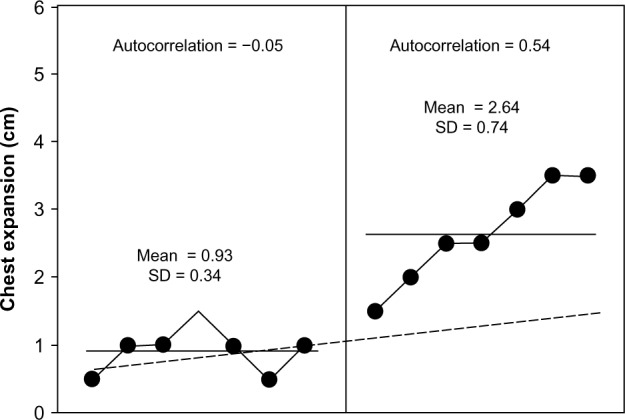
Chest expansion after seven days at baseline (left) and during seven days of rehabilitation by CPT (right). It was evident from the Bloom table that all of the points in the CPT period were above the trend line from the observation period, thus indicating a clinically significant improvement.
Figure 5.
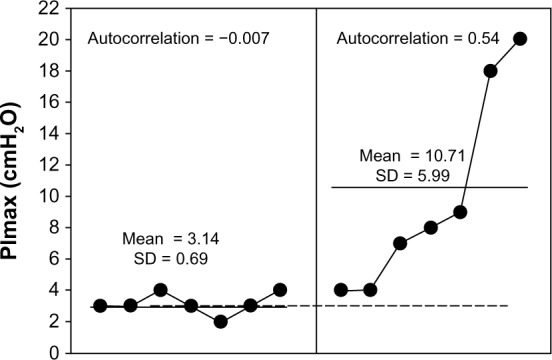
PImax after seven days at baseline (left) and during seven days of rehabilitation by CPT (right). It was evident from the Bloom table that all of the points during rehabilitation by CPT were above the trend line from the observation period, thus indicating a clinically significant change.
The CPT program showed benefit after 7 days by reducing the dyspnea score (Fig. 2) from 5.85 ± 0.69 to 3.85 ± 0.89, as it presented an increasing trend of clinically significant reduction during the CPT period, when all 7 points were below the trend line. This indicated that the reduction of dyspnea score was due to statistical clinical improvement. The analysis of serial dependency or autocorrelation in both periods (baseline and CPT) clearly showed that all of the parameters were not significant; therefore, the benefit presented was directly due to the treatment and not due to disease progression.
The RR showed a significant reduction (Fig. 3). The result showed tachypnea (30 ± 1.68 bpm) and no serial dependency (r = −0.25) during the observation period, while these parameters showed a decreased trend with lower mean (25.5 ± 3.3 bpm) during the CPT period. When analyzing clinical change in the Bloom table, the trend line was seen as 6–7 points below than in the observation period (>90%); therefore, decreasing RR was presented according to the clinical statistics, and the different data were not found to relate to each other (ie, no autocorrelation).
The data on chest expansion during CPT intervention moved higher (Fig. 4; 2.64 ± 0.74 cm) than the aforementioned trend line during the observation period. This clearly demonstrates a significant clinical improvement. When the serial dependency or autocorrelation had been analyzed (r = 0.54) as nonsignificant, the increase in mean values during CPT was evident from the CPT program.
Finally, the result was obtained regarding strength of the respiratory muscles (Fig. 5) when using BreathMax® and elastic bands. The results of this study showed higher PImax (10.71 ± 5.99 cmH2O) when compared to that in the observation period (3.14 ± 0.69 cmH2O). During CPT, PImax data showed a trend that significantly inclined without autocorrelation (r = 0.54).
Furthermore, CPT intervention for seven days could enable the patient to stop using the mechanical ventilator and put spontaneous breathing into effect via a T-piece with O2 flow at 4 Lpm, without the use of any accessory muscle. Although CXR film showed no clinical change in either infiltration or lung volume after seven days of CPT (Fig. 1B), ABG results showed improvement in oxygenation (PaO2 = 85 ± 3.0 mmHg) and ventilation (PaCO2 = 40.0 ± 2.3 mmHg and % SatO2 = 98% ± 2%). In addition, the PaO2/FiO2 ratio changed from 177 in the observation period to 274 at the end of therapy (Table 1). The hematology laboratory showed an improvement in Hb (12.6 mg/dL) and Hct (35.4%), whereas some electrolytes such as sodium, potassium, and chorine or albumin were lower than that in the observation period. Functional ability improved from requiring full support during sitting, standing, and walking at bedside for short periods of only 2 minutes to dependent sitting and standing, and partial ability for longer walking duration (5–10 minutes), with a walker via O2 flow at 4 Lpm. The results were consistent with muscle power improvement from 3 to 4, and the motion of the shoulder joints was within normal range.
Discussion
Scleroderma is a chronic systematic disease involving pulmonary function and relating to respiratory failure, including fatigue of the general muscles of upper and lower limbs.3 Prolonged mechanical ventilation with positive pressure affects the elasticity of the chest wall and thoracic movement directly and causes overinflation of the alveolar within the lung.29,30 Thus, prolonged support from mechanical ventilators directly relates to the respiratory muscle weakness. In addition, commonly found complications in the respiratory system are mucus secretion or retention, poor ventilation or hypoventilation, difficulty in breathing or dyspnea, low exercise tolerance, and poor quality of life.31 Laboratory data found pulmonary impairment, especially in ABG results: respiratory acidosis (pH = 7.30), mild hypoxemia (PaO2 = 74 mmHg), and hypoventilation (PaCO2 = 52 mmHg), including the PaO2/FiO2 ratio of 177, which indicated lung injury or impaired gas diffusion (Table 1). Thus, the ventilator mode was set with a higher oxygen concentration (FiO2 = 0.45) by a pulmonary physician and supported with PEEP (5 cmH2O) for better peripheral alveolar ventilation and oxygenation. Moreover, the hematology test showed low values of Hb (8.5 mg/dL) and Hct (28.5%), which possibly reflect impairment in oxygen-carrying capacity. This finding is the same as that obtained in the CXR investigation, which showed general infiltration in both lower lung fields (Fig. 1A). Therefore, the challenging problems within the lung may be either retained secretion or fibrosis. The investigation discussed earlier presents the pulmonary impairment of ventilation, oxygenation, aerobic capacity, and carrying capacity, which influence functional capacity in both breathing ability and general activities. Fortunately, the case in this study did not show severe impairment of the cardiovascular system, as ECHO screening found no murmur, regular pulse and 50% of ejection fraction, mild aortic valve, and tricuspid valve regurgitation. Nevertheless, evidence of the efficacy of hospitalized rehabilitation with CPT has been reported rarely, ie, whether benefits of CPT during the post-ICU period or recovery stage can be promoted.19 Previous reports that CPT is very important for all patients for secretion clearance, breathing exercise, chest mobilization, cough training, and respiratory muscle training or exercise and to prepare for ceasing use of a mechanical ventilator.9,31 A previous study showed the advantages of CPT for patients in both acute and chronic stages of pulmonary disease,32 as they help them to stop dependency on a mechanical ventilator for removing secretion and increasing pulmonary ventilation. Furthermore, other techniques, such as general exercise training, peripheral and respiratory muscle training, airway clearance, and lung expansion training, have all been shown as beneficial. Original and previous study of the chest mobilization technique helped to improve chest expansion, reduce dyspnea, and improve the expired tidal volume in chronic obstructive pulmonary disease patients.25 Original mobilization techniques have a flexible protocol and include rib mobilization, stretched intercostal muscles, thoracic extension, lateral flexion of the trunk, and rotation of vertebral segments.33 The results in this case study also demonstrate improved chest expansion (Fig. 4) and decreased dyspnea level from 5.85 ± 0.69 to 3.85 ± 0.89 (Fig. 2). The changes in these parameters can be explained as derived benefits, such as airway clearance and improvement in chest wall flexibility, which have direct effects on pulmonary ventilation. Respiratory muscle training is a main goal of rehabilitation while weaning a patient off a mechanical ventilator, and this training relates to RR adaptation of the patient.34 The BreathMax® device is designed and produced commercially in Thailand,26 and its benefits are claimed to increase respiratory muscle strength, remove secretion by bubbling oscillation, and increase humidity in the airway. A previous report showed that the benefit of inspiratory muscle training, as observed in a 79-year-old man after laparotomy, was the patient’s ability to stop using a mechanical ventilator.35 The results of this study confirmed those in a previous review, which proposed the role of CPT as helping patients to stop using or wean themselves off mechanical ventilators.36 This study observed that during 7 days of CPT, RR decreased from 30 ± 1.68 bpm to 25.5 ± 3.3 bpm (Fig. 3) and PImax increased from 3.14 ± 0.69 cmH2O to 10.71 ± 5.99 cmH2O (Fig. 5). This presented better respiratory muscle strength, including reduced dyspnea and RR, which relates to relief of accessory muscle use while breathing.34 In addition, diaphragm movement was observed physically, which related to increased thoracic wall flexibility and improved diaphragmatic muscle function.37 In this study, upper limb exercises were designed in such a way that the major pectoralis muscle was targeted for strengthening. Previous evidence has suggested that upper limb exercise can help to bring about forced inspiration and expiration.38 Thus, both the elastic band exercise and BreathMax® training possibly improve respiratory muscle strength. It was previously reported that an increased PImax indicates the consistent strength of respiratory muscles when relating to intubation in critically ill patients, who were weaned successfully from a mechanical ventilator.39 Besides overall improvement of the pulmonary system, chest expansion, dyspnea, RR, and PImax showed clinically significant change during seven days of CPT rehabilitation. As a result, this analysis had a single-case research design model, and the mean values of all the parameters were found to be above or below the trend line from the baseline, and this was followed by statistical analysis of the protocol using the Bloom table. The changes in each parameter demonstrated no autocorrelation within seven days, which means that their improvement was directly from CPT intervention. However, the results showed nonclinical improvement on CXR film (Fig. 1B), and findings from ABG investigation showed improvement in oxygenation, ventilation, and gas exchange. In addition, the mode of the ventilator was changed from CMV/CPAP to T-piece (Table 1). Regarding the results of functional ability, improvement in walking with less dyspnea was observed, as well as muscle power being found to improve from 3 to 4 during the seven-day rehabilitation period. However, the exercise program in this study was cautious about overexertion during exercise and, therefore, followed the guidelines of the American College of Sport Medicine (ACSM)28 in patients with dyspnea scores from 5 to 10, severe muscle fatigue or pain, headache, blurred vision, changes in significant vital signs (systolic blood pressure of over 250 mmHg and diastolic blood pressure of over 115 mmHg), and a percentage of oxygen saturation of less than 90%. Although this study was performed on a single patient, the results indicated that pulmonary rehabilitation by CPT at the hospital stage is very challenging and should be adapted to the patient’s condition, possibly under the supervision of a rehabilitation physician. The positive results of this study support findings from the previous work by Pitta et al.40 Unfortunately, the patient in this study went missing after being discharged and no follow-up occurred; therefore, sustained and continued effects of rehabilitation could not be investigated in this case.
Conclusion and Limitations
These results demonstrate the possible benefits of short-term pulmonary rehabilitation using CPT in hospital under a single-case research design, which can be applied in severe and stable cases of scleroderma. All programs, namely, airway clearance techniques, respiratory muscle training, general limb exercises, and functional training, should be applied during hospital stay or a critical period. However, the limitations of this study are the short-term period and single patient, thus, a randomized controlled trial study and longer treated period is need in the future to confirm these positive results.
Acknowledgments
All the authors thank the patient, who provided all the treatment results, which present benefits to the health team and other patients with scleroderma.
Footnotes
ACADEMIC EDITOR: Garry Walsh, Editor in Chief of Clinical Medicine Insights: Therapeutics
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 289 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived, designed the protocols, treated, collected data, analyzed the data, and wrote the manuscript: JL. Agreed with manuscript results and conclusions: DP, KW, WE, JK. All the authors approved the final manuscript.
REFERENCES
- 1.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–54. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 2.Calore EE, Cavaliere MJ, Perez NM, Takayasu V, Wakamatsu A, Kill MH. Skeletal muscle pathology in systemic sclerosis. J Rheumatol. 1995;22:2246–9. [PubMed] [Google Scholar]
- 3.Farber HW, Simms RW, Lafyatis R. Care of patients with scleroderma in the intensive care setting. J Intensive Care Med. 2010;25:247–58. doi: 10.1177/0885066610371181. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T. Scleroderma – pathophysiology. Eur J Dermatol. 2009;19:14–24. doi: 10.1684/ejd.2008.0570. [DOI] [PubMed] [Google Scholar]
- 5.Foocharoen C, Siriphannon Y, Mahakkanukaruah A, Suwannaroj S, Nanagara R. Incidence rate and causes of infection in Thai systemic sclerosis patients. Int J Rheum Dis. 2012;15:277–83. doi: 10.1111/j.1756-185X.2012.01728.x. [DOI] [PubMed] [Google Scholar]
- 6.Russell DC, Maloney A, Muir AL. Progressive generalized scleroderma: respiratory failure from primary chest wall involvement. Thorax. 1981;36:219–20. doi: 10.1136/thx.36.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrone A, Barbarossa A, Quacquarelli ME, et al. Pulmonary physiopathology in scleroderma: study of respiratory function in 86 patients. Clin Ter. 2007;158:115–20. [PubMed] [Google Scholar]
- 8.Epstein SK. Decision to extubate. Intensive Care Med. 2002;28:535–46. doi: 10.1007/s00134-002-1268-8. [DOI] [PubMed] [Google Scholar]
- 9.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 10.Iliffe GD, Pettigrew NM. Hypoventilatory respiratory failure in generalized scleroderma. Br Med J. 1983;286:337–8. doi: 10.1136/bmj.286.6362.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shalev T, Haviv Y, Segal E, et al. Outcome of patients with scleroderma admitted to intensive care unit. A report of nine cases. Clin Exp Rheumatol. 2006;24:380–6. [PubMed] [Google Scholar]
- 12.Alkotob ML, Soltani P, Sheatt MA, et al. Reduced exercise capacity and stress-induced pulmonary hypertension in patients with scleroderma. Chest. 2006;130:176–81. doi: 10.1378/chest.130.1.176. [DOI] [PubMed] [Google Scholar]
- 13.Hammon WE. Physical therapy for acutely III patient in the respiratory intensive care unit. In: Irwin S, Tecklin JS, editors. Cardiopulmonary Physical Therapy. 3rd ed. Toronto: Mosby; 1995. pp. 423–44. [Google Scholar]
- 14.Chang AT, Boots RJ, Brown MG, Paratz J, Hodges PW. Reduced inspiratory muscle endurance following successful weaning from prolonged mechanical ventilation. Chest. 2005;128:553–9. doi: 10.1378/chest.128.2.553. [DOI] [PubMed] [Google Scholar]
- 15.Bassi GL, Ferrer M, Marti JD, Comaru T, Torres A. Ventilator-associated pneumonia. Semin Respir Crit Care Med. 2014;35:469–81. doi: 10.1055/s-0034-1384752. [DOI] [PubMed] [Google Scholar]
- 16.Castro AA, Calil SR, Freitas SA, Oliveira AB, Porto EF. Chest physiotherapy effectiveness to reduce hospitalization and mechanical ventilation length of stay, pulmonary infection rate, and mortality in ICU patients. Respir Med. 2013;107:68–74. doi: 10.1016/j.rmed.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Moffat M. Cardiovascular/Pulmonary Essentials, Applying the Preferred Physical Therapist Practice PatternsSM. New Jersy: SLACK Incorporated; 2007. [Google Scholar]
- 18.Ambrosino N, Janah N, Vagheggini G. Physiotherapy in critically ill patients. Rev Port Pneumol. 2011;17:283–8. doi: 10.1016/j.rppneu.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Tang CY, Taylor NF, Blackstock FC. Chest physiotherapy for patients admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease (COPD): a systematic review. Physiotherapy. 2010;96:1–13. doi: 10.1016/j.physio.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Clini E, Ambrosino N. Early physiotherapy in the respiratory intensive care unit. Respir Med. 2005;99:1096–104. doi: 10.1016/j.rmed.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Ottenbacher KJ. Evaluating Clinical Change; Strategies for Occupational and Physical Therapy. Sydney: Williams & Wilkins; 1986. [Google Scholar]
- 22.Sadowsky S, Frownfelter D, Moffat M. Impaired ventilation and respiration/gas exchange associated with respiratory failure (Pattern F) In: Moffat M, editor. Cardiovascular/Pulmonary Essentials; Applying the Preferred Physical Therapist Practice PatternsSM. New Jersy: SLACK Incorporated; 2007. pp. 193–235. [Google Scholar]
- 23.Humberstone N, Teckli JS. Respiratory treatment. In: Irwin S, Tecklin JS, editors. Cardiopulmonary Physical Therapy. 3rd ed. St. Louis, MO: Mosby-Year Book, Inc; 1995. pp. 356–68. [Google Scholar]
- 24.Pryor JA, Prasad AM. Physiotherapy Techniques, Physiotherapy for Respiratory and Cardiac Problems; Adult and Paediatrics. 4th ed. Toronto: Churchill Livingstone; 2008. pp. 137–41. [Google Scholar]
- 25.Leelarungrayub D, Pothongsunun P, Yankai A, Pratanaphon S. Acute clinical benefits of chest wall-stretching exercise on expired tidal volume, dyspnea, and chest expansion in a patient with chronic obstructive pulmonary disease: a single case study. J Bodyw Mov Ther. 2009;13:338–43. doi: 10.1016/j.jbmt.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Padkao T, Watchara B, Chulle J. Conical-PEP is safe, reduces lung hyperinflation and contributes to improve exercise endurance in patients with COPD: a randomised cross-over trial. J Physiother. 2010;56:33–9. doi: 10.1016/s1836-9553(10)70052-7. [DOI] [PubMed] [Google Scholar]
- 27.Hilling L, Smith J. Pulmonary rehabilitation. In: Irwan S, Tecklin JS, editors. Cardiopulmonary Physical Therapy. 3rd ed. Toronto: Mosby; 1995. pp. 445–70. [Google Scholar]
- 28.Armstrong L, Balady GJ, Berry MJ, Davis SE, Davy BM. ACSM’s guidelines for exercise testing and prescription. In: Whaley MH, Brubaker PH, Otto RM, editors. American College of Sports Medicine. 7th ed. Tokyo: Lippincott Williams & Wilkins; 2006. p. 50. [Google Scholar]
- 29.Guerin C, Coussa ML, Eissa NT, et al. Lung and chest wall mechanics in mechanically ventilated COPD patients. J Appl Physiol. 1993;74:1570–80. doi: 10.1152/jappl.1993.74.4.1570. [DOI] [PubMed] [Google Scholar]
- 30.Takeda S, Miyoshi S, Maeda H, et al. Ventilatory muscle recruitment and work of breathing in patients with respiratory failure after thoracic surgery. Eur J Cardiothorac Surg. 1999;15:449–55. doi: 10.1016/s1010-7940(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 31.Hammon WE, Hasson S. Cardiopulmonary Pathophysiology, Principles and Practice of Cardiopulmonary Physical Therapy. 3rd ed. Wiesbaden: Mosby; 1996. pp. 89–90. [Google Scholar]
- 32.Gosselink R. Physical therapy in adults with respiratory disorders: where are we? Rev Bras Fisioter. 2006;10:361–72. [Google Scholar]
- 33.Vibekk P. Chest mobilization and respiratory function. In: Pryor JA, Webber BA, editors. Respiratory Care. Tokyo: Churchill Livingstone; 1991. pp. 103–19. [Google Scholar]
- 34.Capdevila X, Perriqault PF, Ramonatxo M, et al. Changes in breathing pattern and respiratory muscle performance parameters during difficult weaning. Crit Care Med. 1998;26:79–87. doi: 10.1097/00003246-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Bissett B, Leditschke IA. Inspiratory muscle training to endurance weaning from mechanical ventilation. Anaesth Intensive Care. 2007;35:776–9. doi: 10.1177/0310057X0703500520. [DOI] [PubMed] [Google Scholar]
- 36.Ambrosino N, Gabbrielli L. The difficult-to-wean patient. Expert Rev Respir Med. 2010;4:685–92. doi: 10.1586/ers.10.58. [DOI] [PubMed] [Google Scholar]
- 37.Cluzel P, Similowski T, Chartrand-Lefebvre C, Zelter M, Derenne JP, Grenier PA. Diaphragm and chest wall: assessment of the inspiratory pump with MR imaging-preliminary observations. Radiology. 2000;215:574–83. doi: 10.1148/radiology.215.2.r00ma28574. [DOI] [PubMed] [Google Scholar]
- 38.Bolser DC, Reier PJ. Inspiratory and expiratory patterns of the pectoralis major muscle during pulmonary defensive reflexes. J Appl Physiol. 1998;85:1786–92. doi: 10.1152/jappl.1998.85.5.1786. [DOI] [PubMed] [Google Scholar]
- 39.Cader S, Vale R, Dantas E. Effect of inspiratory muscle training on weaning success in critically ill intubated patients. In: Bettany-Saltikov J, editor. Physical Therapy Perspectives in the 21 Century-Challenges and Possibilities. Croatia: INTECH; 2012. pp. 283–304. [Google Scholar]
- 40.Pitta F, Probst V, Garrod R. Pulmonary Rehabilitation in Chronic Respiratory Disease, Physiotherapy for Respiratory and Cardiac Problems; Adult and Paediatrics. 4th ed. Toronto: Churchill Livingstone; 2008. pp. 440–69. [Google Scholar]