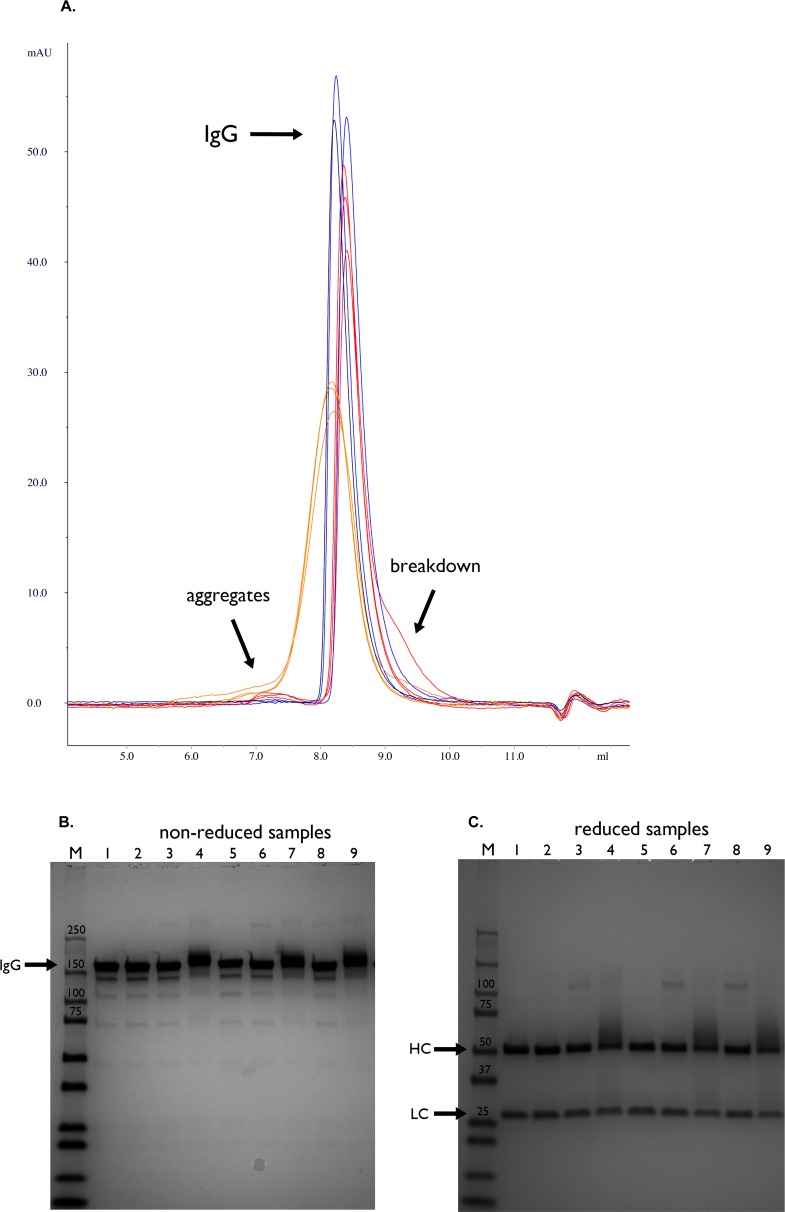
Fig 2.
A. T = 1 month (60°C) gel filtration runs of reconstituted Infliximab samples. Commercial Infliximab (Remicade) stored at 4°C shown in black. Samples dried in sucrose based buffer shown in blue. Samples dried with sucrose-inulin excipients shown in red. Samples dried with sucrose-dextran based excipient shown in orange. B. T = 1 month (60°C) Non-reducing SDS-PAGE analysis of reconstituted Infliximab samples stored at 60°C. M: Dual color protein standard, 1: commercial Infliximab (stored at 4°C), Freeze-dried (vials) 2: sucrose-based (F1), 3: sucrose-inulin (F2), 4: sucrose-dextran (F3), Freeze-dried (Lyoguard trays) 5: sucrose-based (L1), 6: sucrose-inulin (L2), 7: sucrose-dextran (L3), Spray–dried 8: sucrose-inulin (S1), 9: sucrose-dextran (S2). C. T = 1 month (60°C) Reducing SDS-PAGE analysis of reconstituted Infliximab samples stored at 60°C. M: Dual color protein standard, 1: commercial Infliximab (stored at 4°C, Freeze-dried (vials) 2: sucrose-based (F1), 3: sucrose-inulin (F2), 4: sucrose-dextran (F3), Freeze-dried (Lyoguard trays) 5: sucrose-based (L1), 6: sucrose-inulin (L2), 7: sucrose-dextran (L3), Spray–dried 8: sucrose-inulin (S1), 9: sucrose-dextran (S2).