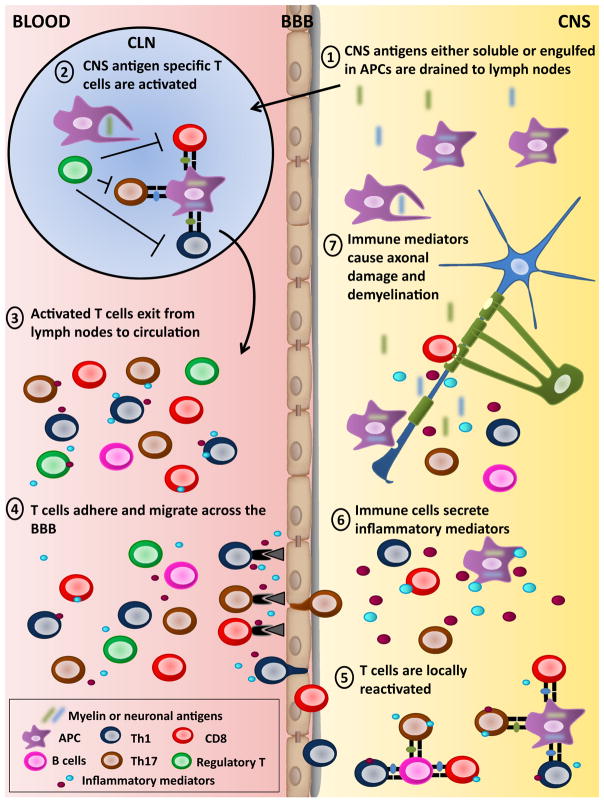
Fig. 1.
Activation and roles of T lymphocytes in the pathogenesis of MS and EAE 1. In contrast to most organs, the brain and spinal cord do not contain defined lymphatic channels; nevertheless, lymphatic drainage for the CSF and the interstitial fluid of the brain parenchyma to the cervical lymph nodes does take place (Laman and Weller 2013). Soluble CNS antigens and professional APCs, such as dendritic cells, that have engulfed myelin or neuronal antigens can travel from the CNS to the cervical lymph nodes (CLN) (Mohammad et al. 2014). 2. Mature APCs that have engulfed myelin or neuronal antigens are detected in cervical lymph nodes obtained from MS patients and EAE animals (Laman and Weller 2013). These APCs can efficiently activate CNS-reactive CD4 and CD8 T lymphocytes. Different regulatory T lymphocyte subsets have been shown to reduce the development and severity of EAE (Kleinewietfeld and Hafler 2014; Sinha et al. 2014). Several groups reported that regulatory T cell subsets from MS patients have impaired regulatory functions compared to healthy donors (Kleinewietfeld and Hafler 2014; Sinha et al. 2014). 3. Activated myelin or neuronal-specific T lymphocytes exit into the peripheral blood to perform immunosurveillance. CNS reactive CD4 and CD8 T lymphocytes obtained from the peripheral blood of MS patients exhibit enhanced activation properties compared to those from health donors. 4. Activated autoreactive T lymphocytes have an enhanced capacity to cross the BBB given their elevated expression of mediators such as chemokine receptors, adhesion molecules, integrins, and cytokines (Goverman 2009; Larochelle et al. 2011). 5. Once in the CNS, T lymphocytes can be reactivated by local APCs (macrophages, microglia and dendritic cells, or B lymphocytes), which are present in human and mouse CNS lesions (Greter et al. 2005; Frohman et al. 2006; Pierson et al. 2014). This antigen-specific reactivation has been shown to be essential to license activated autoreactive T lymphocytes to enter the CNS parenchyma (Bartholomaus et al. 2009). 6. CNS infiltrating Th1, Th17, and CD8 T lymphocytes, and macrophages as well as inflamed microglia secrete soluble mediators (e.g., inflammatory cytokines, free radical, etc.). Moreover, cross-talk between T cells and microglia/macrophages contribute to perpetuate the inflammatory milieu within the CNS. 7. These soluble mediators can injure oligodendrocyte/myelin and axon/neuron structures. Moreover, activated microglia/macrophages can directly phagocyte oligodendrocytes. Similarly, CD8 T lymphocytes have been detected in close proximity to oligodendrocytes and demyelinated axons with polarization of their cytolytic granules (Neumann et al. 2002; Wulff et al. 2003; Lassmann 2004; Saikali et al. 2007). Activated T cells have the capacity to kill oligodendrocytes or neurons (Jurewicz et al. 1998; Sauer et al. 2013; Zaguia et al. 2013). Finally, such damage causes the release of additional CNS antigens that can be further phagocytosed and presented to new waves of CNS-specific T lymphocytes