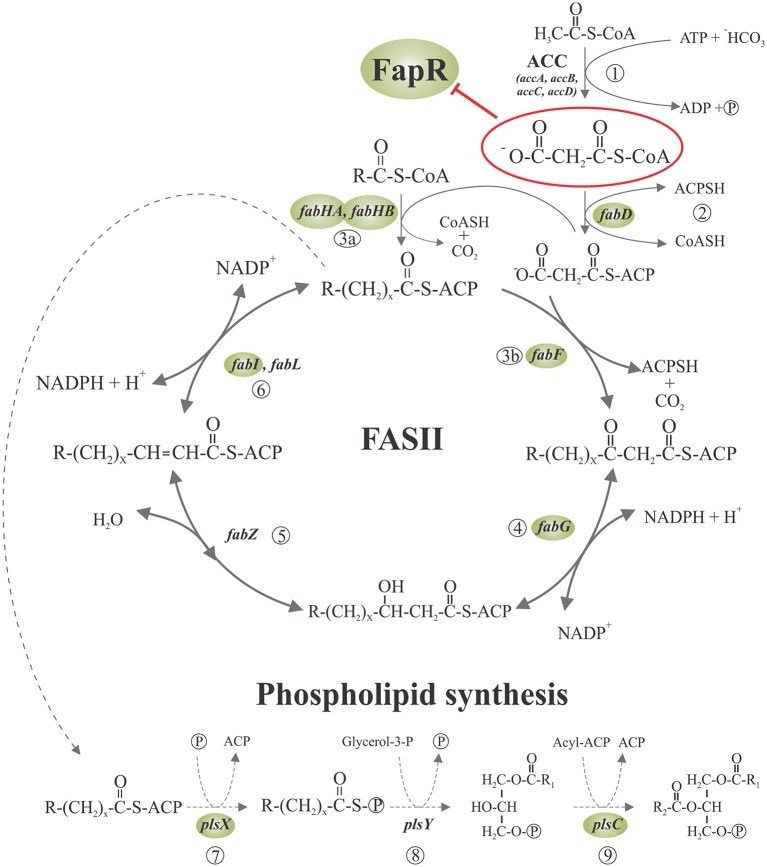
Figure 1.
Fatty acid synthesis and phospholipid initiation steps in Bacillus subtilis. Malonyl-CoA is generated from acetyl-CoA by acetyl-CoA carboxylase (ACC) (1) and then is transferred to ACP by malonyl-CoA transacylase (2). The FabH condensing enzymes initiates the cycles of fatty acid elongation by condensation of acyl-CoA primers with malonyl-ACP (3a). The resultant β-ketoester is reduced by the β-ketoacyl-ACP reductase (4). Then, the β-hydroxyacyl-ACP is dehydrated to the trans-2 unsaturated acyl-ACP by β-hydroxyacyl-ACP dehydrase (5), which is finally reduced by enoyl reductase (6). Subsequent rounds of elongation are initiated by the elongation-condensing enzyme FabF (3b) to generate an acyl-ACP two carbons longer than the original acyl-ACP at the end of each cycle. The long chain acyl-ACP end products of fatty acid synthesis are transacylated in three steps to glycerolphosphate, to generate phosphatidic acid (PA), a key intermediate in the synthesis of phospholipids. First, PlsX catalyzes the synthesis of fatty acyl-phosphate from acyl-ACP (7); then, PlsY transfers the fatty acid from the activated acyl intermediate to the 1-position of glycerol-3-phosphate (8) and finally, lyso-PA is acylated to PA by PlsC (9). Expression of the genes surrounded by shaded ellipses is repressed by the transcriptional regulator FapR, whose activity is, in turn, antagonized by malonyl-CoA (enclosed in a red ellipse). R denotes the terminal group of branched-chain or straight-chain fatty acids. Adapted from Albanesi et al. (2013).