Abstract
Context:
Taurine metabolism disturbance is closely linked to obesity, insulin resistance, and diabetes. Previous evidence suggested that the preventative effects of taurine on diabetes might be through regulating the expression levels of diabetes-related genes.
Objective:
We estimated whether blood taurine levels modified the overall genetic susceptibility to diabetes on improvement of insulin sensitivity in a randomized dietary trial.
Design and Setting:
We genotyped 31 diabetes-associated variants to calculate a genetic risk score (GRS) and measured plasma taurine levels and glycemic traits among participants from the Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) trial.
Participants:
Seven-hundred eleven overweight or obese participants (age 30–70 y; 60% females) had genetic variants genotyped and blood taurine levels measured.
Intervention:
Participants went on 2-year weight-loss diets, which were different in macronutrient composition.
Main Outcome Measure:
Improvements in glycemic traits were measured.
Results:
We found that baseline taurine levels significantly modified the effects of diabetes GRS on changes in fasting glucose, insulin, and homeostatic model assessment of insulin resistance (HOMA-IR) during the 2-year diet intervention (P-interaction = .04, .01, .002, respectively), regardless of weight loss. High baseline taurine levels were associated with a less reduction in both glucose and HOMA-IR among the participants with the lowest tertile of diabetes GRS (both P = .02), and with a greater reduction in both insulin and HOMA-IR among those with the highest tertile of diabetes GRS (both P = .04).
Conclusions:
Our data suggest that blood taurine levels might differentially modulate the effects of diabetes-related genes on improvement of insulin sensitivity among overweight/obese patients on weight-loss diets.
We studied whether blood taurine levels interacted with diabetic genetic susceptibility in a trial and found taurine modified the effects of diabetes genetic risk score on changes in glycemic traits.
Taurine, 2-amino ethanesulfonic acid, is the most abundant free amino acid and accounts for approximately 0.1% of total body weight. Taurine can be acquired exogenously from food such as meat and sea food, as well as be endogenously synthesized (with low capacity) from methionine and cysteine (1). Taurine metabolism is closely related to obesity, insulin resistance, and diabetes. Previous experimental studies have demonstrated the potential benefits of taurine supplementation on glycemic control and insulin resistance in animal models (3, 4). In humans, although the effectiveness of dietary taurine supplementation on diabetes patients is still controversial (5, 6), circulating taurine levels were consistently decreased in diabetic subjects (7–9). Urinary taurine release as a measure of dietary taurine intake was inversely related to cardiovascular risk factors in a large epidemiology study (2).
Growing evidence has suggested that the beneficial effects of taurine might be through modification of gene expression on obesity (10–12), lipid metabolism (13, 14), and diabetes (15, 16). In addition, it has been proposed that genetic susceptibility may interact with metabolic status in affecting disease risk (17). Thus far, no human studies have assessed the interplay between genetic susceptibility and circulating taurine levels on diabetes risk.
In the present study, we quantified the association of plasma taurine levels with improvement of insulin resistance in the context of dietary interventions varying in macronutrients, among participants from a large, 2-year randomized dietary intervention trial, the Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) trial. We further assessed the modification effects of taurine on type 2 diabetes (T2D) genetic predisposition.
Materials and Methods
Study design and population
The POUNDS Lost trial is a randomized dietary intervention trial to compare the effects on body weight of energy-reduced diets that differed in compositions of fat, protein, and carbohydrate. The trial was conducted at the Harvard T.H. Chan School of Public Health and Brigham and Women's Hospital, Boston; and the Pennington Biomedical Research Center of the Louisiana State University System, Baton Rouge, from 2004 through 2007. The study design, methods, and primary results have been described in detail elsewhere (18). In brief, 811 overweight or obese adults (age, 51 ± 9 y; females, 64%; white, 79%; body mass index, 33 ± 4 kg/m2) were randomly assigned to one of four diets, with targeted percentages of energy derived from fat, protein, and carbohydrates respecitvely in the four diets of: 20, 15, and 65%; 20, 25, and 55%; 40, 15, and 45%; and 40, 25, and 35%. After 2 years of intervention, 645 (80%) completed the study. The study was approved by the local human subjects committees. All participants in the POUNDS Lost trial provided written informed consent; written informed consents for the present study were not required further.
In the POUNDS Lost trial, body weight and waist circumference were measured in the morning before breakfast at baseline, 6 months, and 2 years. Fasting blood samples were obtained at baseline, 6 months, and 2 years. Analyses of glucose and insulin were performed at the Clinical Laboratory at Pennington, and glucose and insulin were measured using an immunoassay with chemiluminescent detection on the Immulite analyzer (Diagnostic Products Corporation). Insulin resistance was estimated by homeostasis model assessment of insulin resistance (HOMA-IR) calculated as [fasting insulin (μU/mL) × fasting glucose (mg/dL)]/(18.01 × 22.5); and insulin secretion was estimated by homeostasis model assessment of β-cell function (HOMA-B) as [363 × fasting insulin (μU/mL)]/[fasting glucose (mg/dL)] −63.5] (19). Race was self reported. The plasma taurine concentration was quantified in April 2014 using fasting plasma samples that had been stored at −80°C since collection. The samples were prepared and analyzed by the Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University Hospital Leipzig, Germany, by using electrospray tandem-mass spectrometry. The detailed procedures are published elsewhere (20, 21).
Genotyping
DNA was extracted from the buffy coat fraction of centrifuged blood by using the QIAmp Blood Kit (QIAGEN). We selected 31 single-nucleotide polymorphisms (SNPs) previously associated with T2D at a genome-wide significance level in Caucasians (22). For each individual, we summed the number of risk alleles of the SNPs to produce an unweighted genetic risk score (GRS) (23). The SNPs included in the GRS (Supplemental Table 1) were genotyped successfully in 734 of 811 total participants using the OpenArray SNP Genotyping System (BioTrove). The genotype success rates were approximately 99% in available DNA samples. Replicated quality control samples (10%) were included in every genotyping plate with greater than 99% concordance (24). In the present study, we included 711 participants who had both genetic data and plasma taurine levels measured at baseline.
Statistical analysis
The primary outcomes for current analysis were changes in fasting glucose, insulin, insulin resistance, and insulin secretion during the intervention period. We log transformed plasma levels of taurine, insulin, HOMA-IR, and HOMA-B to improve their distributions of imperfect normality. Baseline data are presented as mean (± SD) for the continuous variables and numbers (and percentages) for the categorical variables. Baseline characteristics were compared using χ2 tests for categorical variables and using general linear models for continuous variables across the groups of participants based on their baseline taurine levels. The effects of genetic factors and diet intervention on outcomes at 6 months and 2 years were analyzed using generalized estimating equations (GEE) method. Covariates adjustment included age, sex, race, dietary group, intervention time, and the baseline value for the respective outcome in Model 1. Model 2 further adjusted for concurrent weight loss. Potential taurine-GRS interactions or taurine-diet interactions were tested by including the taurine-by-GRS or taurine-by-diet group multiplicative term in the GEE models, respectively.
In a secondary analysis, we also tested whether the genetic effects on improvement in insulin resistance significantly changed during the intervention periods by different groups of baseline taurine levels using linear mixed models. In sensitivity analyses we analyzed the associations among the white participants (∼80% of all participants) only, and the results were similar to that in the whole population (data not shown).
All reported P values were two sided, and P < .05 was considered statistically significant. All statistical analyses were performed in SAS version 9.4 (SAS Institute). This trial was registered at ClinicalTrials.gov as trial number NCT00072995.
Results
Characteristics of study population at baseline
Baseline characteristics of participants across tertiles of baseline taurine levels are presented in Table 1. The median levels of taurine across tertiles were 1.19, 1.63, and 2.09 μmol/L. The distribution of the genetic predisposition and dietary intervention group assignments of study participants, who were overweight or obese, was not statistically different across tertiles of taurine levels (all P > .05).
Table 1.
Baseline Characteristics of the Study Participants in the POUNDS Lost (n = 711) Across Tertiles of Baseline Taurine Levels
| Tertiles of Baseline Taurine Levels |
|||
|---|---|---|---|
| T1 (n = 237) | T2 (n = 237) | T3 (n = 237) | |
| Age, y | 50.44 ± 9.11 | 51.46 ± 9.16 | 50.86 ± 9.57 |
| Female, n (%) | 152 (64.1) | 132 (55.7) | 151 (63.7) |
| Ethnic group, n (%) | |||
| White | 188 (79.3) | 186 (78.5) | 193 (81.4) |
| Black | 36 (15.2) | 39 (16.5) | 33 (13.9) |
| Hispanic, Asian, or other | 13 (5.5) | 12 (5.1) | 11 (4.6) |
| Low-fat diets, n (%) | 138 (58.2) | 110 (46.4) | 121 (51.1) |
| Average-protein diets, n (%) | 117 (49.4) | 124 (52.3) | 134 (56.5) |
| T2D GRS, n of risk alleles | 34.05 ± 4.21 | 33.86 ± 4.19 | 34.09 ± 4.75 |
| Baseline taurine, μmol/L | 1.19 (0.3) | 1.63 (0.2) | 2.09 (0.3) |
| Height, cm | 168.00 ± 8.77 | 169.12 ± 8.81 | 168.74 ± 8.83 |
| Baseline BMI, kg/m2 | 32.34 ± 3.63 | 32.69 ± 4.03 | 32.92 ± 3.93 |
| Baseline weight, kg | 91.65 ± 14.9 | 93.86 ± 15.77 | 94.12 ± 15.84 |
| Baseline fasting glucose, mg/dL | 91.22 ± 12.16 | 92.78 ± 12.13 | 91.28 ± 10.69 |
| Baseline fasting insulin, μU/L | 9.80 (6.5) | 10.60 (9.7) | 11.10 (9.1) |
| Baseline HOMA-IR | 2.17 (1.7) | 2.48 (2.4) | 2.51 (2.2) |
| Baseline HOMA-B | 125.33 (95.9) | 131.20 (98.7) | 144.77 (124.9) |
Abbreviations: BMI, body mass index; T1, tertile 1; T2, tertile 2; T3, tertile 3.
Data are presented as mean ± sd or n (%), except for taurine, fasting insulin, HOMA-IR, and HOMA-B presented as median (interquartile range).
The χ2 test was used for categorical variables and general linear regression with covariates of age (except for age), sex (except for sex), and weight (except for weight) for continuous variables (except for baseline taurine). No statistical significant results were found (all P > .05).
Main effects of plasma taurine and genetic predisposition on improvement of insulin resistance
The main association between baseline taurine and changes in insulin resistance did not reach statistical significance level in our study sample (β = −0.0125; P = .47) after adjustments of age, sex, race, dietary groups, time of the intervention, and baseline level of HOMA-IR.
The ranges of number of risk alleles in the lowest, middle, and highest tertile of GRS, which was built on 31 SNPs were 15–31, 32–35, and 36–62, respectively. Supplemental Figure 1 presents the distribution of T2D GRS and its associations with changes in glucose, insulin, HOMA-IR, and HOMA-B among the white participants. We observed that a higher T2D GRS was associated with less reduction in both insulin resistance (P = .03) and glucose (P = .008) during the diet intervention.
Interaction between baseline plasma taurine level and genetic predisposition on change of insulin resistance
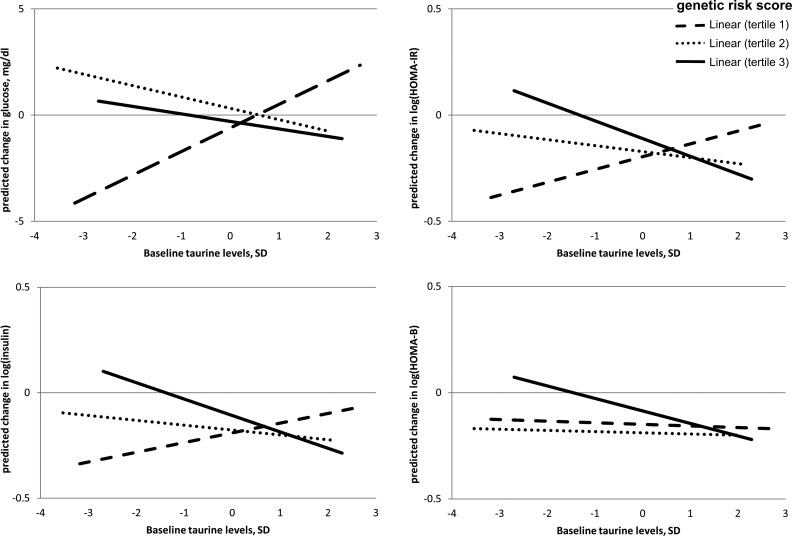
Table 2 shows the associations of baseline taurine with changes in weight, fasting glucose, fasting insulin, insulin resistance calculated as HOMA-IR, and insulin secretion calculated as HOMA-B across the tertiles of T2D GRS using repeated measures of outcomes at 6 months and 2 years. The median baseline taurine levels across T2D GRS tertiles were 1.65, 1.58, and 1.63 μmol/L, respectively (P value for linear trend = .47). Model 1 adjusted for age, sex, race, time during the intervention, and the baseline value for respective outcome; and Model 2 additionally adjusted for concurrent weight loss. Across these two models, the overall associations were similar, and the significant taurine-GRS interaction on changes in glucose, insulin, and HOMA-IR remained after adjusting for concurrent weight change. Figure 1 shows the associations of baseline taurine with predicted changes in these glycemic traits by GRS tertiles in Model 2. In Model 2, baseline taurine levels were associated with a less reduction in both fasting glucose and HOMA-IR among the participants with the lowest tertile of T2D GRS (both P = .02), and with a greater reduction in both insulin and HOMA-IR among those with the highest tertile of T2D GRS (both P = .04), during the 2-year period of intervention (P value for taurine-GRS interaction = .04, .01, and .002 for changes in glucose, insulin, and HOMA-IR respectively; Table 2). No significant taurine-GRS interaction on changes in HOMA-B was detected.
Table 2.
Associations of Baseline Taurine Levels with Changes in Body Weight, Glucose, Insulin, HOMA-IR, and HOMA-B Across Tertiles of Diabetes Genetic Risk Score
| Baseline taurine, μmol/L | Diabetes Genetic Risk Score |
P Interaction | |||||
|---|---|---|---|---|---|---|---|
| Tertile 1 (Lowest) |
Tertile 2 |
Tertile 3 (Highest) |
|||||
| 1.65 (0.7) |
1.58 (0.6) |
1.63 (0.7) |
|||||
| Outcome traits | β ± se | P | β ± se | P | β ± se | P | |
| Model 1 | |||||||
| Weight change, kg | −0.15 ± 0.44 | .73 | −0.47 ± 0.38 | .21 | −0.28 ± 0.41 | .49 | .87 |
| Change in glucose, mg/dL | 1.00 ± 0.47 | .03 | −0.61 ± 0.42 | .15 | −0.47 ± 0.57 | .41 | .04 |
| Change in log-insulin | 0.04 ± 0.03 | .14 | −0.02 ± 0.03 | .40 | −0.06 ± 0.03 | .03 | .006 |
| Change in log-HOMA-IR | 0.05 ± 0.03 | .06 | −0.03 ± 0.03 | .32 | −0.07 ± 0.03 | .03 | .004 |
| Change in log-HOMA-B | −0.02 ± 0.03 | .47 | −0.004 ± 0.03 | .89 | −0.05 ± 0.03 | .08 | .57 |
| Model 2 | |||||||
| Change in glucose, mg/dL | 1.01 ± 0.45 | .02 | −0.33 ± 0.43 | .44 | −0.35 ± 0.50 | .48 | .04 |
| Change in log-insulin | 0.04 ± 0.02 | .08 | 0.006 ± 0.02 | .78 | −0.05 ± 0.03 | .04 | .01 |
| Change in log-HOMA-IR | 0.05 ± 0.02 | .02 | 0.004 ± 0.02 | .87 | −0.06 ± 0.03 | .04 | .002 |
| Change in log-HOMA-B | −0.02 ± 0.03 | 0.46 | 0.02 ± 0.03 | .55 | −0.04 ± 0.03 | .17 | .68 |
Data are median (interquartile range) for the first row and β ± se for the rest of the rows, representing adjusted association size for each SD of baseline level of taurine with change in each outcome trait.
Model 1 adjusted for age, sex, race, dietary group, follow-up time, and the baseline value for the respective outcome trait, and Model 2 is further adjusted for concurrent weight change.
Figure 1.
Associations of predicted changes in glucose, insulin, insulin resistance, and insulin secretion with baseline taurine levels, by tertiles of diabetes GRS. P values were adjusted for age, sex, race, dietary group, follow-up time, concurrent weight change, and baseline respective value (all P values for taurine-GRS interaction < .05).
Trajectory changes in insulin resistance
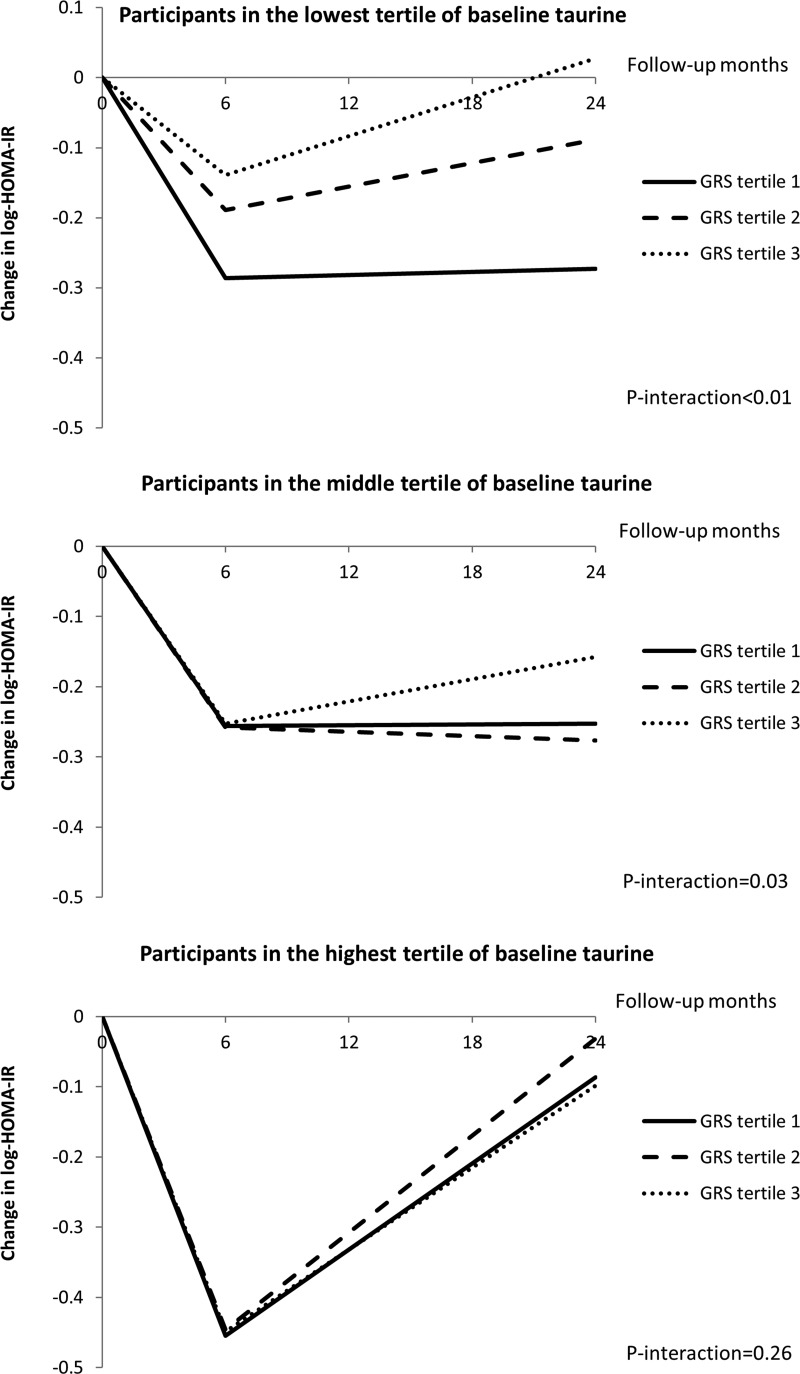
We then used linear mixed models to assess the trajectory of changes in insulin resistance during the intervention period by different taurine and GRS groups. As previously reported (18), insulin resistance showed the greatest improvement at 6 months, and later regressed toward the baseline levels at 2 years. Such a regression may be partly due to reduced compliance to intervention and weight regain during this period. Among the participants who had the lowest and middle tertiles of baseline taurine levels, the association of diabetes genetic predisposition with improvement of insulin resistance changed significantly during the 2-year intervention (both P values for GRS-time interaction < .05; Figure 2). And among those who had the highest tertile of baseline taurine levels, such associations did not significantly change during the intervention period (P for GRS-time interaction = .26; Figure 2).
Figure 2.
Trajectory changes in insulin resistance by tertiles of baseline taurine levels and diabetes genetic predisposition. Adjustments include age, sex, race, dietary group, concurrent weight change, and baseline HOMA-IR value. P-interaction indicates the P value for interaction of diabetes GRS and follow-up months on change in HOMA-IR.
Discussion
In a 2-year randomized weight-loss dietary intervention trial, we found that baseline plasma levels of taurine significantly interacted with the T2D genetic predisposition on improvement of insulin resistance. Our finding shows that among individuals who have a higher genetic risk of diabetes, those with a higher level of baseline circulating taurine might benefit more in improving insulin resistance from weight-loss diets compared with those with a lower level of taurine; whereas among individuals who have a lower genetic risk of diabetes, a lower level of baseline circulating taurine may be more beneficial compared with a higher level of taurine. No interaction between taurine and T2D genetic predisposition was found on improvement of insulin secretion.
Our observations that baseline blood taurine levels modified the effects of diabetes genetic factors on changes of insulin resistance in humans are novel; however, they are in line with the long-standing concept of gene-metabolite interactions (17, 25). The potential mechanisms for the beneficial effects of taurine on diabetic pathogenesis include modulating phosphorylation states of Insulin receptor substrate 1 (which contributes to our GRS) (26) and the levels of peroxisome proliferator-activated receptor-α (which is a nuclear receptor transcript factor affecting fatty acid metabolism) (27), interaction with insulin receptor to improve insulin sensitivity (26, 28), antioxidation/inflammation (29), and regulating the expression levels of diabetes-related genes (9, 15, 16, 30). Of note, these regulated genes include peroxisome proliferator-activated receptor-γ (PPARG) (31), which is included in our T2D GRS (Supplemental Table 1). The diverse mechanisms through which metabolites tune gene expression have been described, and they include riboswitches, direct interactions with transcription factors, regulation of cofactors, chromatin remodeling, chromatin modification, and hormone signaling (32). The levels where the cellular pathways regulated by the T2D genetic factors and those indicated/induced by circulating taurine levels interact remain to be determined. However, based on the aforementioned evidence, it is reasonable to postulate that the observed taurine-GRS interaction on improvement of insulin resistance may reflect, at least partially, the regulation effect of plasma taurine on diabetes-related gene expression.
Another possibility is that the anti-obesity action of taurine (29) may mediate the gene-taurine interaction on improvement of insulin resistance. The finding that in our study the observed gene-taurine interaction effect was only moderately affected by and independent of concurrent weight change is against this hypothesis. We acknowledge that further experimental and clinical data are warranted to clarify such functional mechanisms.
We estimated a genetic score to evaluate the overall susceptibility to diabetes based on 31 well-established T2D-predisposing variants identified from genome-wide association studies, and as expected we found that a higher genetic risk was related to a less improvement of glycemic control and insulin resistance caused by weight-loss diets. And among the participants with a lower level of taurine (ie, the two lower taurine tertiles), the improvement of insulin resistance was greater for those with a lower genetic risk (ie, the two lower GRS tertiles) than those with a higher genetic risk (Figure 2). Such observations favor the clinical implications of personalized medicine. Conversely, the eventual clinical practice must weigh the costs of the genetic and/or metabolomic testing, against the effectiveness of these individualized treatments, especially in high-risk populations.
Circulating taurine levels are partially controlled by the biosynthesis rate in the liver where taurine is also used in the biosynthesis of taurine conjugated bile acids. It is possible that dietary fat may influence the circulating taurine levels via modulating bile acid excretion. Besides, previous animal models revealed dietary amino acids may affect taurine metabolism (33). However, we did not observe significant dietary effects (effects from either the overall dietary intervention or the different dietary intervention arms) on circulating taurine levels (data not shown), or significant interactions between taurine and dietary macronutrient composition on improvement of insulin resistance. In addition, we did not observe an interaction between taurine and diabetes genetic susceptibility on insulin secretion, although previous studies suggested that taurine might improve pancreatic islet function (34, 35).
To our knowledge, the present study is the first to investigate interactions of circulating taurine levels with overall diabetes genetic predisposition on changes in insulin resistance in a long-term randomized diet-intervention trial. The study design allowed for reliable control for potential confounding. Analysis of repeated measures of outcomes at multiple time points improved study power. Besides, the GEE model is fairly robust to the choices of the correlation structure and flexible for missing data compared with other models (36).
Nevertheless, we acknowledge that the current study might be underpowered to detect moderate association between taurine and insulin resistance or interactions between taurine and diet. In addition, replication in separate diet intervention trials is needed to verify our findings. Besides, generalization of this study might be limited to whites who are overweight or obese, because 80% of our population is white.
In conclusion, we reported an interaction between baseline blood taurine levels and diabetes genetic predisposition on change in insulin resistance in response to 2-year weight-loss dietary interventions. Our data suggest that populations with different levels of circulating taurine might benefit differently in improving insulin resistance from weight-loss dietary intervention based on their genetic background of diabetes.
Acknowledgments
We are thank all participants in the trial for their dedication and contribution to the research.
The authors' responsibilities were as follows: Y.Z. designed the study, analyzed data, and wrote the manuscript; U.C. contributed to the acquisition of amino acid data and reviewed the manuscript; T.H., T.W., Y.H., W.M., G.A.B., J.T., and F.M.S. contributed to discussion and edited and reviewed the manuscript; L.Q. designed the study, reviewed data, contributed to discussion, and edited and reviewed the manuscript. L.Q. is the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The POUNDS LOST trial was supported by grants from the National Heart, Lung, and Blood Institute (HL073286) and the General Clinical Research Center, National Institutes of Health (RR-02635). This study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL126024, and HL034594); the Boston Obesity Nutrition Research Center (DK46200); the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383); the National Natural Science Foundation of China (NNSFC30972453); and the Program for New Century Excellent Talents in University (NCET-10–0420). Dr Lu Qi was a recipient of an American Heart Association Scientist Development Award (0730094N). These funders have no role in the study design, implementation, analysis, or interpretation of the data.
Disclosure Summary: The authors have nothing to disclose.
This study was registered in ClinicalTrials.gov as trial number NCT00072995.
- GEE
- Generalized Estimating Equations
- GRS
- genetic risk score
- HOMA-IR
- homeostasis model assessment of insulin resistance
- HOMA-B
- homeostasis model assessment of β-cell function
- POUNDS Lost trial
- Preventing Overweight Using Novel Dietary Strategies trial
- PPARG
- peroxisome proliferator-activated receptor-γ
- SNPs
- single nucleotide polymorphisms
- T2D
- type 2 diabetes.
References
- 1. Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S. mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism. 2002;51:1191–1197. [DOI] [PubMed] [Google Scholar]
- 2. Sagara M, Murakami S, Mizushima S, et al. Taurine in 24-h urine samples is inversely related to cardiovascular risks of middle aged subjects in 50 populations of the world. Adv Exp Med Biol. 2015;803:623–636. [DOI] [PubMed] [Google Scholar]
- 3. Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol. 1999;77:749–754. [PubMed] [Google Scholar]
- 4. Nakaya Y, Minami A, Harada N, Sakamoto S, Niwa Y, Ohnaka M. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am J Clin Nutr. 2000;71:54–58. [DOI] [PubMed] [Google Scholar]
- 5. Brøns C, Spohr C, Storgaard H, Dyerberg J, Vaag A. Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes mellitus. Eur J Clin Nutr. 2004;58:1239–1247. [DOI] [PubMed] [Google Scholar]
- 6. Xiao C, Giacca A, Lewis GF. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia. 2008;51:139–146. [DOI] [PubMed] [Google Scholar]
- 7. Franconi F, Bennardini F, Mattana A, et al. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: Effects of taurine supplementation. Am J Clin Nutr. 1995;61:1115–1119. [DOI] [PubMed] [Google Scholar]
- 8. Merheb M, Daher RT, Nasrallah M, Sabra R, Ziyadeh FN, Barada K. Taurine intestinal absorption and renal excretion test in diabetic patients: A pilot study. Diabetes Care. 2007;30:2652–2654. [DOI] [PubMed] [Google Scholar]
- 9. Ito T, Schaffer SW, Azuma J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids. 2012;42:1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liaset B, Madsen L, Hao Q, et al. Fish protein hydrolysate elevates plasma bile acids and reduces visceral adipose tissue mass in rats. Biochim Biophys Acta. 2009;1791:254–262. [DOI] [PubMed] [Google Scholar]
- 11. Lin S, Hirai S, Yamaguchi Y, et al. Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol Nutr Food Res. 2013;57:2155–2165. [DOI] [PubMed] [Google Scholar]
- 12. Kim KS, Ji HI, Chung H, et al. Taurine chloramine modulates the expression of adipokines through inhibition of the STAT-3 signaling pathway in differentiated human adipocytes. Amino Acids. 2013;45:1415–1422. [DOI] [PubMed] [Google Scholar]
- 13. Murakami S, Yamagishi I, Asami Y, et al. Hypolipidemic effect of taurine in stroke-prone spontaneously hypertensive rats. Pharmacology. 1996;52:303–313. [DOI] [PubMed] [Google Scholar]
- 14. Yokogoshi H, Mochizuki H, Nanami K, Hida Y, Miyachi F, Oda H. Dietary taurine enhances cholesterol degradation and reduces serum and liver cholesterol concentrations in rats fed a high-cholesterol diet. J Nutr. 1999;129:1705–1712. [DOI] [PubMed] [Google Scholar]
- 15. Carneiro EM, Latorraca MQ, Araujo E, et al. Taurine supplementation modulates glucose homeostasis and islet function. J Nutr Biochem. 2009;20:503–511. [DOI] [PubMed] [Google Scholar]
- 16. Sirdah MM. Protective and therapeutic effectiveness of taurine in diabetes mellitus: A rationale for antioxidant supplementation. Diabetes Metab Syndr. 2015;9:55–64. [DOI] [PubMed] [Google Scholar]
- 17. Doria A, Wojcik J, Xu R, et al. Interaction between poor glycemic control and 9p21 locus on risk of coronary artery disease in type 2 diabetes. JAMA. 2008;300:2389–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 20. Brauer R, Leichtle A, Fiedler G, Thiery J, Ceglarek U. Preanalytical standardization of amino acid and acylcarnitine metabolite profiling in human blood using tandem mass spectrometry. Metabolomics. 2011;7:344–352. [Google Scholar]
- 21. Ceglarek U, Muller P, Stach B, Buhrdel P, Thiery J, Kiess W. Validation of the phenylalanine/tyrosine ratio determined by tandem mass spectrometry: Sensitive newborn screening for phenylketonuria. Clin Chem Lab Med. 2002;40:693–697. [DOI] [PubMed] [Google Scholar]
- 22. Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qi Q, Meigs JB, Rexrode KM, Hu FB, Qi L. Diabetes genetic predisposition score and cardiovascular complications among patients with type 2 diabetes. Diabetes care. 2013;36:737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: The Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation. 2011;124:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roede JR, Uppal K, Park Y, Tran V, Jones DP. Transcriptome-metabolome wide association study (TMWAS) of maneb and paraquat neurotoxicity reveals network level interactions in toxicologic mechanism. Toxicol Rep. 2014;1:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maturo J, Kulakowski EC. Taurine binding to the purified insulin receptor. Biochem Pharmacol. 1988;37:3755–3760. [DOI] [PubMed] [Google Scholar]
- 27. Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids. 2016;48:549–558. [DOI] [PubMed] [Google Scholar]
- 28. Wu N, Lu Y, He B, et al. Taurine prevents free fatty acid-induced hepatic insulin resistance in association with inhibiting JNK1 activation and improving insulin signaling in vivo. Diabetes Res Clin Pract. 2010;90:288–296. [DOI] [PubMed] [Google Scholar]
- 29. Imae M, Asano T, Murakami S. Potential role of taurine in the prevention of diabetes and metabolic syndrome. Amino Acids. 2014;46:81–88. [DOI] [PubMed] [Google Scholar]
- 30. Murakami S. Role of taurine in the pathogenesis of obesity. Mol Nutr Food Res. 2015;59:1353–1363. [DOI] [PubMed] [Google Scholar]
- 31. Tsuboyama-Kasaoka N, Shozawa C, Sano K, et al. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology. 2006;147:3276–3284. [DOI] [PubMed] [Google Scholar]
- 32. Ladurner AG. Rheostat control of gene expression by metabolites. Mol Cell. 2006;24:1–11. [DOI] [PubMed] [Google Scholar]
- 33. Trautwein EA, Hayes KC. Amino acid interaction with taurine metabolism in cats. Adv Exp Med Biol. 1992;315:15–22. [DOI] [PubMed] [Google Scholar]
- 34. Ribeiro RA, Santos-Silva JC, Vettorazzi JF, et al. Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic β-cells. Amino Acids. 2012;43:1791–1801. [DOI] [PubMed] [Google Scholar]
- 35. Ribeiro RA, Santos-Silva JC, Vettorazzi JF, Cotrim BB, Boschero AC, Carneiro EM. Taurine supplementation enhances insulin secretion without altering islet morphology in non-obese diabetic mice. Adv Exp Med Biol. 2015;803:353–370. [DOI] [PubMed] [Google Scholar]
- 36. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]