Abstract
Background
Biocontrol agents are regarded as promising and environmental friendly approaches as agrochemicals for phytodiseases that cause serious environmental and health problems. Trichoderma species have been widely used in suppression of soil-borne pathogens. In this study, an endophytic fungus, Trichoderma gamsii YIM PH30019, from healthy Panax notoginseng root was investigated for its biocontrol potential.
Methods
In vitro detached healthy roots, and pot and field experiments were used to investigate the pathogenicity and biocontrol efficacy of T. gamsii YIM PH30019 to the host plant. The antagonistic mechanisms against test phytopathogens were analyzed using dual culture, scanning electron microscopy, and volatile organic compounds (VOCs). Tolerance to chemical fertilizers was also tested in a series of concentrations.
Results
The results indicated that T. gamsii YIM PH30019 was nonpathogenic to the host, presented appreciable biocontrol efficacy, and could tolerate chemical fertilizer concentrations of up to 20%. T. gamsii YIM PH30019 displayed antagonistic activities against the pathogenic fungi of P. notoginseng via production of VOCs. On the basis of gas chromatography-mass spectrometry, VOCs were identified as dimethyl disulfide, dibenzofuran, methanethiol, ketones, etc., which are effective ingredients for antagonistic activity. T. gamsii YIM PH30019 was able to improve the seedlings' emergence and protect P. notoginseng plants from soil-borne disease in the continuous cropping field tests.
Conclusion
The results suggest that the endophytic fungus T. gamsii YIM PH30019 may have a good potential as a biological control agent against notoginseng phytodiseases and can provide a clue to further illuminate the interactions between Trichoderma and phytopathogens.
Keywords: antagonistic activity, biocontrol agents, mycoparasitism, Panax notoginseng, Trichoderma gamsii
1. Introduction
Panax notoginseng (Burk.) F.H. Chen (Araliaceae), an important member of the genus Panax [1], is a valued traditional Chinese medicinal herb. Because of its low adaptive capacity, which strictly depends on climate and environment, P. notoginseng is found in middle and high elevation areas around the subtropical zone [2], mainly in Wenshan, Yunnan, China. With increasing demand in pharmaceutical industries, P. notoginseng has been domestically cultured on a large scale [3]. However, severe soil-borne diseases have significantly limited the yield and quality of P. notoginseng. The root-rot disease caused by a plethora of phytopathogens [4], [5], such as Fusarium solani, Fusarium oxysporum, Phoma herbarum, and Rhizoctonia solani [6], [7], is the most destructive among the phytodiseases of notoginseng, and is thought to be a main cause for the continuous cropping obstacles for P. notoginseng. To combat the disease and increase output, chemical fertilizers and pesticides have been extensively applied. These agro approaches resulted in soil salinization [8], [9], changes in soil microbial biodiversity, and environmental pollution. Hence, there is an urgent need for sustainable and ecologically safe ways to control P. notoginseng diseases.
Biological control, which includes the use of specific organisms to control phytopathogens, is a nature-friendly ecological approach. Trichoderma spp. distributed widely in soil have been developed as a source of biocontrol agents for years. They are remarkable for their rapid growth, utilization of diverse substrates, resistance to biotic and abiotic stresses, and promotion of plant growth [10], [11], [12]. Some Trichoderma species show effective antagonistic activities toward plant pathogens, such as Fusarium spp., Phoma (Pleospora) betae, and Rhizoctonia solani [13], [14], with the mechanisms of mycoparasitism, competition for nutrition and space, and indirect inhibition through volatile organic compounds (VOCs) [15].
VOCs can permeate and travel in soil pores for long distances [16]. VOCs emitted by Trichoderma spp. are crucial in controlling plant pathogens [17], [18], activating plant immunity, and enhancing plant growth [19]. These potential biological values and features have attracted the attention of researchers in recent years [20], [21], [22], [23]. However, Trichoderma species, substrates, and nutrient conditions can influence their biocontrol viability [24], [25]. In screening antagonistic microbes against notoginseng diseases from the bioniches of P. notoginseng, an endophytic fungus with mycoparasitic activity was obtained from a healthy notoginseng root. In this study, experiments were carried out to estimate: (1) the pathogenicity of Trichoderma gamsii YIM PH30019 to its host plant; (2) biocontrol efficacy to phytodiseases in the continuous field experiments; (3) tolerance capacity to chemical fertilizers; (4) mycoparasitism and induced VOCs antagonistic activities against phytopathogens; (5) VOCs identified with gas chromatography-mass spectrometry (GC-MS).
2. Materials and methods
2.1. Microorganism culture
T. gamsii YIM PH30019 was isolated from the root of a healthy 2-y-old P. notoginseng plant collected in July 2012 from Wenshan, China. The root samples were thoroughly washed with running water to remove soil particles, treated with Tween 20 for 1 h, sterilized with 70% ethanol for 1 min, and finally washed with sterilized distilled water for three times. Roots were crushed in an autoclaved mortar and pestle. The paste was serially diluted to 10−4 with sterilized distilled water, and a 1-mL dilution was coated on the plate containing 20 mL potato dextrose agar (PDA) medium. The discrete colonies were transferred and purified on fresh PDA plates. Identification was based on morphological and ITS molecular phylogenetic analysis. The ITS sequence of T. gamsii was submitted to GenBank with the accession no. KP715352. The pathogenic fungi—Phoma herbarum (YIM PH30340), Fusarium flocciferum (YIM PH30355), Scytalidium lignicola (YIM PH30094), and Epicocum nigrum (YIM PH30306)—used in this study were isolated from the rotten root of P. notoginseng. Their pathogenicity was confirmed using the method described by Miao et al [6]. All strains were maintained on PDA medium, and the voucher specimens were preserved at Yunnan Institute of Microbiology, Kunming, China.
The deactivated cell walls of E. nigrum, S. lignicola, P. herbarum, and F. flocciferum were prepared according to Yang et al [26] with modifications. Briefly, a 6-mm mycelial disk was cut from the edges of actively growing colonies of test pytopathogens and transferred into a 500-mL conical flask containing 200 mL potato dextrose broth (PDB) medium. The flasks were incubated on a shaker at 28°C, 180 rpm for 7 d, then mycelia were collected by filtering the culture broth. Cell walls were lyophilized and powdered. Antagonistic activities caused by VOCs were tested on the deactivated cell wall agar medium (DCWA) (15.0 g deactivated cell walls, 6.9 g NaH2PO4, 2.0 g KH2PO4, 1.4 g (NH4)2SO4, 1.0 g peptone, 0.3 g MgSO4·7H2O, 0.3 g urea, and 15.0 g agar in 1 L distilled water, pH not adjusted), and VOCs were collected by culturing T. gamsii YIM PH30019 in the deactivated cell wall broth medium (DCWB). Controls were inoculated in the above media without the deactivated cell walls.
2.2. Pathogenic test of T. gamsii YIM PH30019
The pathogenic abilities of T. gamsii YIM PH30019 was estimated with 1-y-old healthy P. notoginseng in greenhouse tests. Three P. notoginseng seedlings were planted in pots with the size of 10 L (0.6 × 0.5 × 0.125 m, L/W/H) containing 6 L sterilized soil. Spores of T. gamsii YIM PH30019 were collected from the PDA plates. A 0.2-mL spore solution (1 × 1012) of T. gamsii YIM PH30019 was applied to the soil around roots. The treatment without inoculation with YIM PH30019 was set as control. Six replications were used for treatment and control. The pots were incubated at 25°C for 2 mo, and P. notoginseng plants were collected to check the virulence of YIM PH30019.
2.3. Biocontrol estimation of root-rot disease
The biocontrol efficacy of T. gamsii YIM PH30019 was estimated in vitro with detached healthy notoginseng roots. YIM PH30019, pathogenic fungi, and the mixture of YIM PH30019 and test pathogenic fungus were inoculated with a healthy root in a pot containing 400 g soil autoclaved at 121°C for 60 min. Four replicates were set for each treatment. The pots were kept in a shed similar to the field-planting condition. Spores of T. gamsii and test pathogenic fungi were collected from PDA plates. Ten milliliter spores (1.0 × 1010) of T. gamsii YIM PH30019, pathogenic fungus, and the mixture of T. gamsii YIM PH30019 and pathogenic fungus were added into the autoclaved soil and mixed together thoroughly. The surface-sterilized healthy notoginseng roots were laid into the soil at a depth of 5 cm. Roots treated with pathogenic fungus or T. gamsii YIM PH30019 were set as controls. The biocontrol efficacy of PH30019 and virulence of test phytopathogens were checked every week for 1 mo (Fig. 1 in Support Information).
Disease coverage of type 0, 1, 2, 3, and 4 lesions for each P. notoginseng root was evaluated following indicators [27] with modification, where 0 = no lesions, 1 = one to several lesions (roots blacking < 25%), 2 = extensive lesions or several entire roots necrotic (25–50% roots blacking), 3 = lesions on roots and darkening of crown (50–75% root blacking), 4 = extensive darkening of crown (75–100% roots blacking).
2.4. Tolerance capacity to chemical fertilizers
To determine the effect of chemical fertilizers on the growth of T. gamsii YIM PH30019, three chemical fertilizers were selected—ammonium chloride, potassium nitrate, and ammonium dihydrogen phosphate—and mixed at a ratio of 1:1:1 (w/w/w). A 6-mm plug of actively growing T. gamsii YIM PH30019 was placed on the center of a petri dish containing 25 mL PDA medium amended with chemical fertilizers at the following concentrations (w/v): 0%, 2%, 4%, 6%, 8%, 10%, 12%, 14%, 16%, 18%, 20%, 22%, 24%, 26%, 28%, and 30%. Each concentration was repeated thrice. The petri dishes were incubated at 28°C for 1 wk. The morphology and the growth diameter were recorded daily.
2.5. Dual cultures and observations of mycoparasitism
The antagonism of T. gamsii YIM PH30019 against pathogens was investigated with dual culture tests [28]. The mycoparasitism of T. gamsii YIM PH30019 was observed by collecting the hyphae from the interaction zone between T. gamsii and test phytopathogens. The hyphal sample was transferred to glass coverslips, fixed with 2.5% glutaraldehyde, dehydrated in a series of ascending ethanol concentrations (50–100%) (v/v), dried in desiccator, and coated with gold in a sputter-coater (SCD 005; BAL-TEC, Switzerland); then, it was examined with a scanning electron microscope (Quanta 200FEG; FEI Company, Hillsboro, Oregon, USA).
2.6. Antagonistic assay of VOCs from T. gamsii YIM PH30019 against pathogens
The antagonistic effect of VOCs to pathogens was evaluated using the method described by Dennis and Webster [17] with modifications. A 6-mm plug of T. gamsii YIM PH30019 was cut from the actively growing cultures, then placed on the center of a 90-mm petri dish containing 25 mL DCWA. The lid of plates with YIM PH30019 was replaced by the bottom containing PDA inoculated with pathogenic fungus. The dishes were taped together with parafilm. T. gamsii YIM PH30019 grown on the medium without deactivated cell walls of phytopathogens was used as a control. The plates were incubated at 28°C, and the radial growth of test pathogens was observed and compared daily for 1 wk.
2.7. VOC analysis by GC-MS
For the identification of VOCs from T. gamsii YIM PH30019, spore suspension was prepared to a final concentration of 5 × 10mL−1 in sterile water. A 200-μL spore suspension was transferred into a 15-mL conical flask containing 5 mL DCWB medium. The flasks were incubated on a shaker at 28°C, 180 rpm for 4 d. Extraction was carried out at 50°C for 30 min with preconditioned PA fiber (85μM, polyacrylate) in the headspace. The VOCs were desorbed by placing the fiber into the GC injection port for 1 min at 250°C. Compounds were resolved in the following conditions: helium flow, 1.0 mL/min; oven temperature, 50°C (2 min), 6°C/min to 180°C (1 min), then 6°C/min to 260°C (5 min); and mass spectrometer monitoring in full scan mode (m/z 35–550) operated in the electron ionization mode at 70 eV with a source temperature of 220°C. Compounds were tentatively identified by the mass spectra using the National Institute of Standards and Technology database.
2.8. Biocontrol efficacy in continuous cropping field
Field experiments were conducted in a continuous cropping artificial shed in which crops of P. notoginseng harvested in the previous year were used. The experimental field is located at Xiangshuilong Village, a traditional notoginseng cultivation center in Wenshan, Yunnan. Rice bran was mixed with equal mass water, and autoclaved at 121°C, for 60 min. T. gamsii YIM PH30019 was inoculated in the autoclaved rice bran until the final T. gamsii YIM PH30019 spores concentration was up to 1.0 × 1010 spore/g. The field soil was treated with chemical fumigants as described by Gao et al [29] prior to planting notoginseng. Fermented rice bran with T. gamsii YIM PH30019 was applied to each plot (treated with Trichoderma, Tt) (1.3 × 2.0 m) as the basal fertilizer with the use of 0.15 kg/m2, and 112 1-y-old healthy notoginseng seedlings were planted according to the description of Sun et al [30] in January 2015. Experimental plots applied only with autoclaved rice bran were set as controls (CK). Treatment (Tt) and control (CK) (Fig. 2 in Support Information) were conducted in triplicate. After the emergence of notoginseng, 0.5 mL spore (1 × 105 spores/mL) of T. gamsii YIM PH30019 was applied to the soil by root irrigation for further protection. Seedling emergence and dead seedling rate were recorded from the emergence of notoginseng.
3. Results
3.1. Pathogenicity to P. notoginseng
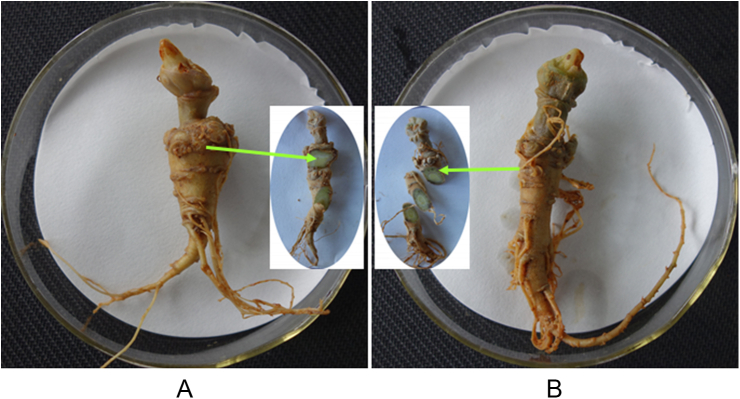
T. gamsii YIM PH30019 showed no pathogenic activity (Fig. 1). After coinoculation for 2 mo, either treated with T. gamsii YIM PH30019 or the control, P. notoginseng plants maintained their healthy growing status. The roots showed no symptoms on the surface and inner tissues (Fig. 1). These results suggested that the endophytic T. gamsii YIM PH30019 cannot cause root-rot disease, and is harmless to its host plant.
Fig. 1.
Pathogenicity estimation of Trichoderma gamsii YIM PH30019 to Panax notoginseng plants in 2 mo. (A) The root in control. (B) The root inoculated with T. gamsii YIM PH30019.
3.2. Biocontrol estimation of root-rot disease
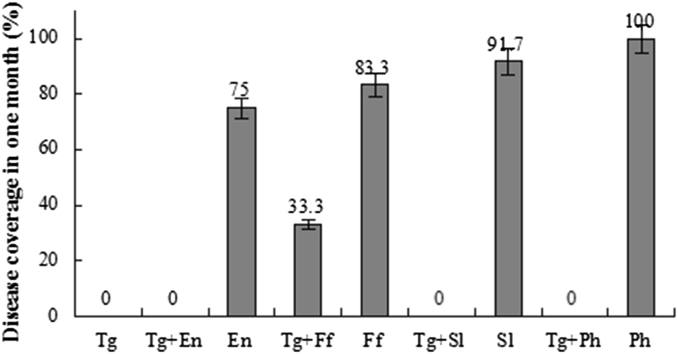
T. gamsii YIM PH30019 presented favorable biocontrol efficacy in vitro as per evaluation in 1 mo (Fig. 1 in Support Information). All the treatments inoculated with pathogens showed severe rot-root symptoms and up to lesion type 4 on the roots. Two detached roots treated with T. gamsii YIM PH30019 and F. flocciferum displayed rot symptom at lesion type 1 level. Roots inoculated with T. gamsii YIM PH30019 and other three pathogenic fungi remained healthy during the observation period. Disease coverage again indicated the severe pathogenicity of four pathogens (Fig. 2; Fig. 1 in Support Information), and presented the desirable biocontrol potential of T. gamsii YIM PH30019 against the root-rot disease of P. notoginseng.
Fig. 2.
In vitro biocontrol estimation of Trichoderma gamsii YIM PH30019 against phytopathogens of Panax notoginseng for 1 mo. Negative control is treatment with the inoculation of four pathogens, and positive control is treatment inoculated with T. gamsii YIM PH30019. All results were calculated based on four replications. En, Epicocum nigrum; Ff, Fusarium flocciferum; Ph, Phoma herbarum; Sl, Scytalidium lignicola; Tg, Trichoderma gamsii.
3.3. Effect of chemical fertilizers
The effect chemical fertilizers on the growth and morphology of T. gamsii YIM PH30019 was observed in a series concentrations of chemical fertilizers (Table 1). T. gamsii YIM PH30019 could grow in concentration levels of up to 20% chemical fertilizers. The strain grew well at concentrations ranging from 0% to 12% without differences in mycelial morphology. When the composite fertilizer concentration was above 12%, the growth rate became slower. At the same time, the mycelial morphology changed from cottony to flat, and the volatile smell disappeared gradually as the concentration increased. At 20% composite fertilizer, T. gamsii YIM PH30019 grew in crystalline particles, and not in a radial pattern. The mycelia in higher chemical fertilizer concentrations (from 14% to 20%) faded away within several days.
Table 1.
Chemical fertilizers' effect on the growth of Trichoderma gamsii YIM PH300191)
| Concentration (% w/v) | Inoculation days (mycelium diameter, mm)2) |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 0 | 24.9 ± 0.3 | 60.2 ± 0.3 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 |
| 2 | 19.9 ± 0.4 | 51.6 ± 0.1 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 |
| 4 | 18.6 ± 0.1 | 40.1 ± 0.2 | 68.0 ± 0.3 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 |
| 6 | 14.0 ± 0.1 | 32.4 ± 0.2 | 54.1 ± 0.20 | 77.1 ± 0.1 | 90.0 ± 0.0 | 90.0 ± 0.0 | 90.0 ± 0.0 |
| 8 | 12.5 ± 0.8 | 19.4 ± 0.4 | 42.2 ± 0.1 | 63.1 ± 0.1 | 81.1 ± 0.1 | 90.0 ± 0.0 | 90.0 ± 0.0 |
| 10 | 11.5 ± 0.1 | 16.2 ± 0.1 | 37.0 ± 0.2 | 55.0 ± 0.2 | 69.0 ± 0.1 | 83.1 ± 0.2 | 90.0 ± 0.0 |
| 12 | 9.0 ± 0.2 | 12.0 ± 0.2 | 31.0 ± 0.2 | 45.0 ± 0.2 | 55.2 ± 0.2 | 67.3 ± 0.2 | 75.2 ± 0.1 |
| 14 | —3) | 9.1 ± 0.2 | 22.9 ± 0.2 | 34.1 ± 0.2 | 42.2 ± 0.1 | 50.1 ± 0.2 | 59.0 ± 0.2 |
| 16 | — | — | 14.1 ± 0.2 | 23.4 ± 0.2 | 32.1 ± 0.2 | 39.1 ± 0.2 | 45.1 ± 0.2 |
| 18 | — | — | 10.0 ± 0.2 | 17.1 ± 0.1 | 22.1 ± 0.2 | 24.2 ± 0.2 | 29.6 ± 0.2 |
| 20 | — | — | — | — | 10.1 ± 0.3 | 13.1 ± 0.2 | 15.2 ± 0.1 |
| 22 | — | — | — | — | — | — | — |
| 24 | — | — | — | — | — | — | — |
| 26 | — | — | — | — | — | — | — |
| 28 | — | — | — | — | — | — | — |
| 30 | — | — | — | — | — | — | — |
Data were recorded for a 7-d inoculation.
Colony diameter was an average value of three replicates.
No growth was observed in our tests.
3.4. Dual culture and mycoparasitism
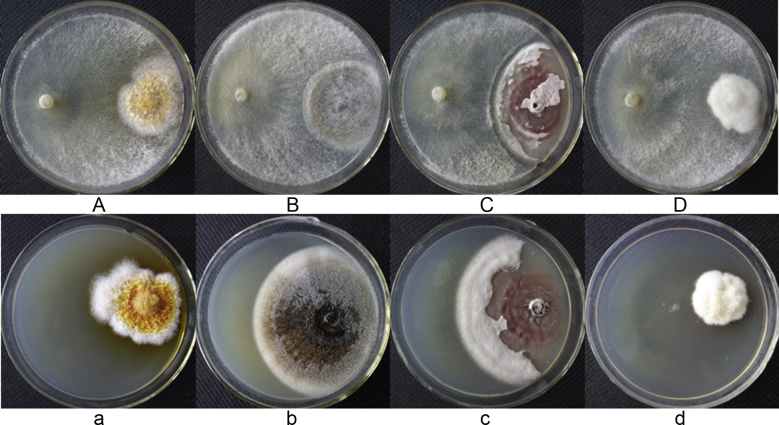
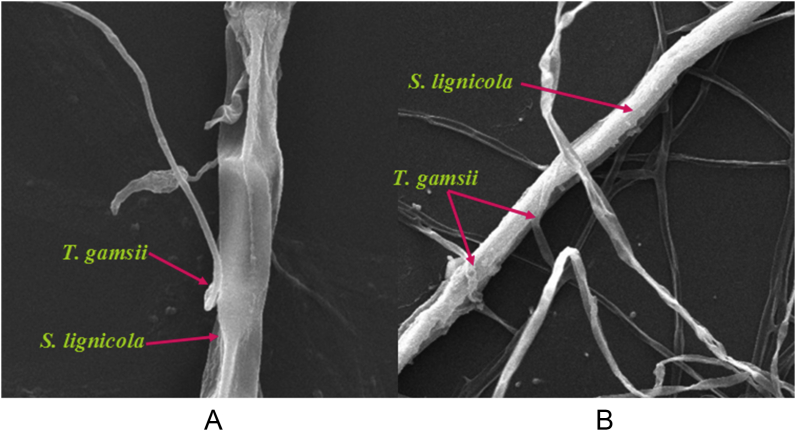
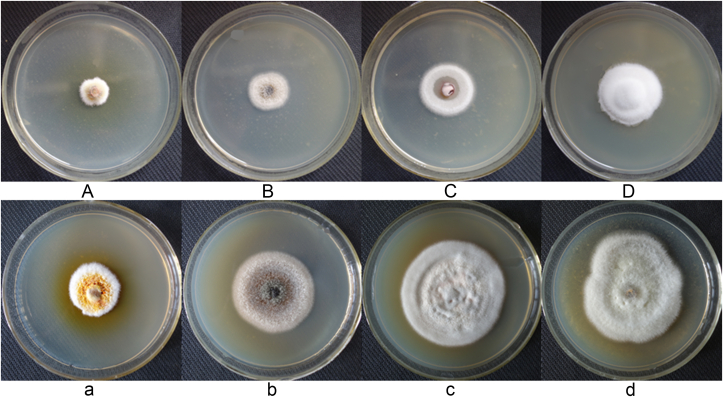
T. gamsii YIM PH30019 grew quickly and covered the test pathogens in 3 d. The pathogenic fungi were confined in very small colonies with a curled edge (Fig. 3), and mycelia withered gradually after 5 d of cocultivation. The pathogens grew well in control (without T. gamsii YIM PH30019), and the mycelia were flourishing with pigments. In scanning electron microscopy (SEM), the hypae of T. gamsii YIM PH30019 grew alongside, circled and coiled around the pathogenic fungal hyphae (Fig. 4), which are the typical mycoparasitic characteristics of Trichoderma.
Fig. 3.
Mycoparasitism of Trichoderma gamsii YIM PH30019 to phytopathogenic fungi of Panax notoginseng at 5 d. (A) Epicocum nigrum (B) Scytalidium lignicola (C) Phoma herbarum (D) Fusarium flocciferum; (a), (b), (c), and (d) are the corresponding control of (A), (B), (C), and (D), respectively.
Fig. 4.
Scanning electron micrographs (Quanta 200FEG; FEI Company, USA) of mycoparasitism of Trichoderma gamsii YIM PH30019 to Scytalidium lignicola at 7 d. (A) Recognizing. (B) Coiling.
3.5. Inhibitory activity of VOCs produced by T. gamsii YIM PH30019
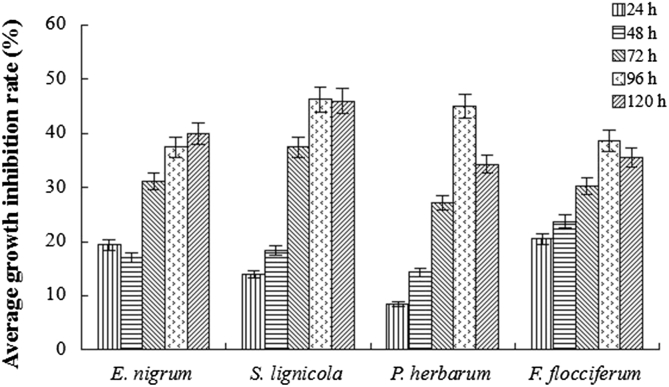
Compared with the control, the VOCs induced by the deactivated cell walls of pathogenic fungi showed significant suppression on the growth of test phytopathogens. The pathogenic radical growth in the experimental group was much smaller than that in the control (Fig. 5). The highest inhibition rate appeared on the 4th d (Fig. 6), reaching 45.0%, 37.5%, 38.6%, and 46.3% for E. nigrum, S. lignicola, P. herbarum, and F. flocciferum, respectively.
Fig. 5.
Antagonistic activity of volatile organic compounds (VOCs) produced by Trichoderma gamsii YIM PH30019 against tested phytopathogens (at 96 h). (A) Epicocum nigrum (B) Scytalidium lignicola (C) P. herbarum (D) Fusarium flocciferum; (a), (b), (c), and (d) are the corresponding control of (A), (B), (C), and (D), respectively.
Fig. 6.
Growth inhibition rate of phytopathogens by volatile organic compounds (VOCs) produced by Trichoderma gamsii YIM PH30019.
3.6. Identification of VOCs
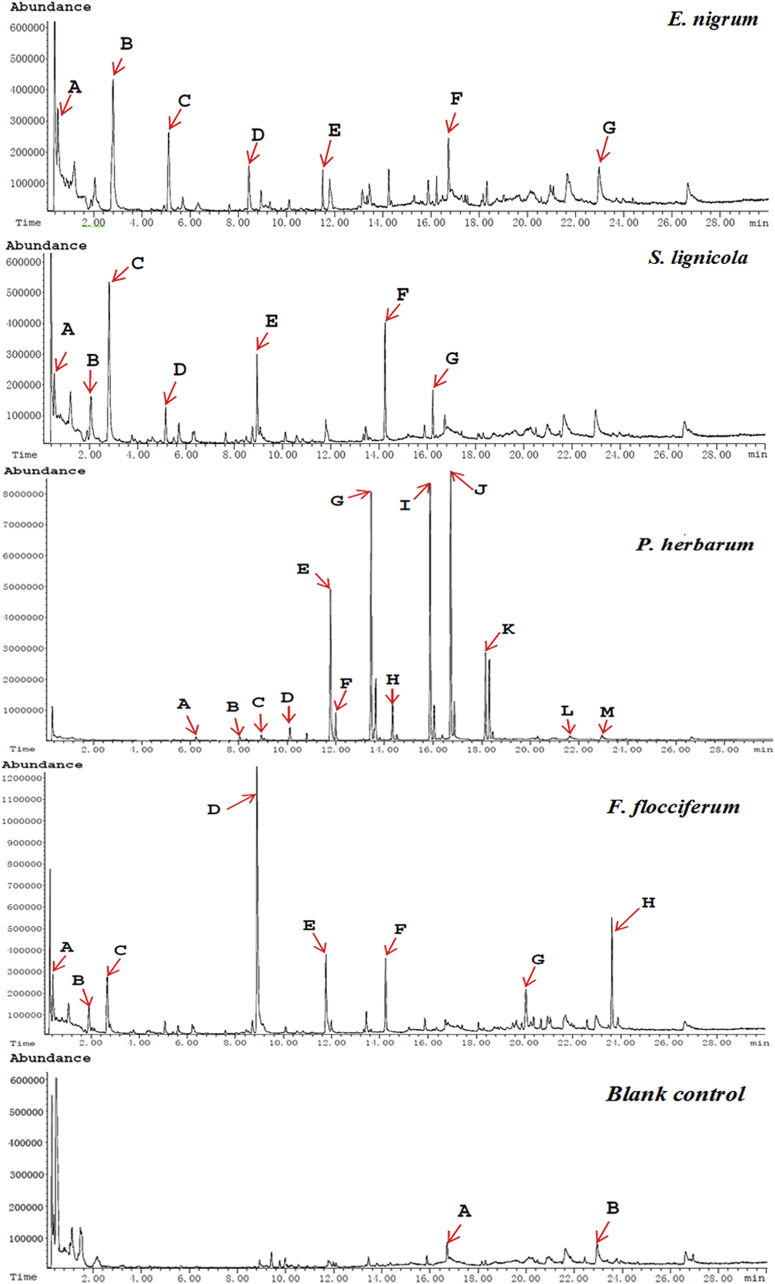
The VOCs were identified using GC-MS after 96 h of cultivation (Fig. 7), and are listed in Table 2. The profiles of the volatiles were obviously different among the test phytopathogens, and the amounts of VOCs were greater than those in the control. A total of 22 volatile compounds were identified (marked with capital letters A–M in Fig. 7), wherein substances, such as dimethyl disulfide, dibenzofuran, methanethiol, and ketones, may serve as chemical inhibitors to the growth of pathogenic fungi.
Fig. 7.
The induced volatile organic compounds (VOC) profiles produced by Trichoderma gamsii YIM PH30019. VOCs were collected from T. gamsii inoculated in four deactivated cell wall broth (DCWB) media and control without the deactivated cell walls, respectively. Peaks of compounds were recorded and are identified in Table 2.
Table 2.
The induced VOCs produced by Trichoderma gamsii YIM PH30019
| Treatments1) | Retention time (min) | Peak | Volatile compounds |
|---|---|---|---|
| Deactivated cell walls of Epicocum nigrum | 0.514 | A | Methanethiol |
| 2.811 | B | Dimethyl disulfide | |
| 5.114 | C | 2-Hexanone, 4-methyl | |
| 8.445 | D | Mesitylene | |
| 11.505 | E | 3-Heptanone, 5-ethyl-4-methyl | |
| 16.725 | F | 2-Undecanone | |
| 22.967 | G | Fluorene | |
| Deactivated cell walls of Scytalidium lignicola | 0.517 | A | Methanethiol |
| 2.047 | B | Methyl thiolacetate | |
| 2.799 | C | Dimethyl disulfide | |
| 5.146 | D | Butanethioic acid, S-methyl ester | |
| 8.936 | E | 3-Octanone | |
| 14.241 | F | Benzene, 2-methoxy-4-methyl-1-(1-methylethyl) | |
| 16.220 | G | N,N,N′-Trimethyl-1,4-phenylenediamine | |
| Deactivated cell walls of Phoma herbarum | 6.246 | A | 2-Heptanone |
| 8.004 | B | 2-Heptanone,6-methyl-6-methyl- | |
| 8.924 | C | 3-Octanone | |
| 10.107 | D | β-Phellandrene | |
| 11.783 | E | 2-Nonanone | |
| 11.996 | F | 2-Nonanol | |
| 13.452 | G | 2-Decanone | |
| 14.331 | H | 2-Decanone | |
| 15.884 | I | 2-Undecanone | |
| 16.731 | J | 2-Undecanone | |
| 18.154 | K | 2-Dodecanone | |
| 21.628 | L | Dibenzofuran | |
| 22.954 | M | Fluorene | |
| Deactivated cell walls of Fusarium flocciferum | 0.508 | A | Methanethiol |
| 1.931 | B | Methyl thiolacetate | |
| 2.797 | C | Dimethyl disulfide | |
| 8.914 | D | 3-Octanone | |
| 11.780 | E | 2-Nonanone | |
| 14.234 | F | Benzene, 2-methoxy-4-methyl-1-(1-methylethyl) | |
| 20.096 | G | Bicyclo[5.2.0]nonane | |
| 23.633 | H | 5H-Inden-5-one | |
| Control2) | 15.873 | A | 2-Undecanone |
| 22.954 | B | Fluorene |
DCWB, deactivated cell wall broth; VOCs, volatile organic compounds.
T. gamsii YIM PH30019 was inoculated in the DCWB medium.
T. gamsii YIM PH30019 was inoculated in the above medium without deactivated cell wall.
3.7. Biocontrol efficacy in continuous cropping field
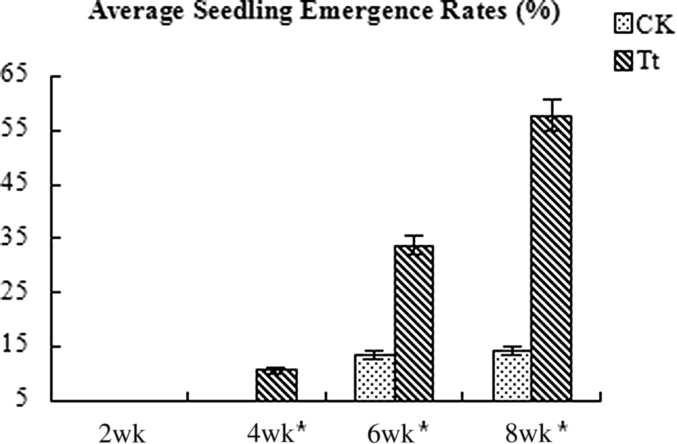
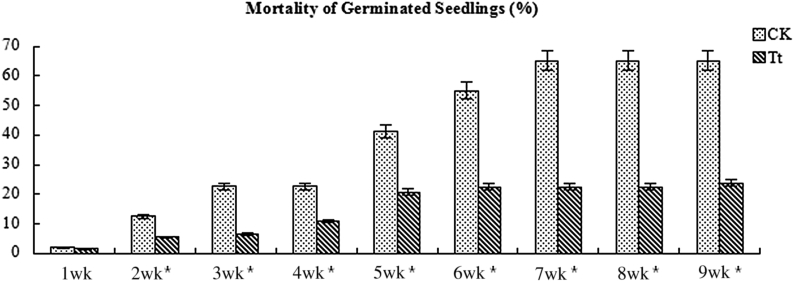
Emergence of seedling in the continuous cropping field is very important in notoginseng planting. In our experiments, the average emergence rate in the treatments reached up to 57.8%, much more than that in the control (average 14.2%) at the end of March 2015 (Fig. 8). In the observation, dead seedlings occurred during the emergence of notoginseng, which was mainly caused by root-rot disease, and were removed from the field after they were recorded. Until the end of May 2015, the average percent mortality in the treatment plots was 23.9% and 61.3% in the controls (Fig. 9), indicating that T. gamsii YIM PH30019 may have protective effects on notoginseng seedlings against root-rot disease in the field experiments (Fig. 2 in Support Information). In early May, a small-scale disease breakout caused the mortalities to increase up to 20% in both the treatment plots and the controls. After root irrigation with T. gamsii YIM PH30019, the seedling death rate in treatment became significantly lower than that in CK.
Fig. 8.
Average Panax notoginseng seedling emergence rates in continuous cropping field. Results are expressed as mean ± SD (n = 3). *Statistically significant differences (Duncan's multiple range test, p < 0.05). CK, control; SD, standard deviation; Tt, treatment plot with T. gamsii YIM PH30019.
Fig. 9.
Seedlings mortality until May 31, 2015. Trichoderma gamsii YIM PH30019 was applied to the treatment plots by root-irrigation in the 5th wk. Results are expressed as mean ± SD (n = 3). *Statistically significant differences (Duncan's multiple range test, p < 0.05). CK, control; SD, standard deviation; Tt, treatment plot with T. gamsii YIM PH30019.
4. Discussion
Biocontrol is the most ecofriendly approach to the management of plant disease. Further understanding of the biocontrol mechanisms from different aspects is the critical role for agricultural applications in the future [31]. The most important genus used as a biocontrol agent is the Trichoderma [32], which has an outstanding interaction with plant and plant pathogens [33]. The interactions include antagonism toward fungal pathogens, plant growth promotion, plant defense responses, and protection of plants from environmental stresses, such as salinity and drought [34], [35]. Continuous cropping obstacles significantly affect P. notoginseng seedling emergence and cause severe mortality to seedlings [36]. In our field experiments with continuous cropping soil, T. gamsii YIM PH30019 showed desirable biocontrol potential compared to plots without inoculation of T. gamsii YIM PH30019 (Fig. 2 in Support Information).
Frequent use of chemical fertilizers results in soil salinization and can also cause loss of protective efficacy for some biocontrol agents [37], [38]. To achieve higher yield, multiple chemical fertilizers are widely used in notoginseng planting. Potential microbial agents should be hyperosmotic in controlling the diseases of P. notoginseng. The hyperosmotic property was evaluated with a series of composite chemical fertilizers. The results showed that T. gamsii YIM PH30019 can grow well at 0–12% (w/v) and bear up to 20% (w/v) of chemical fertilizers that are usually used in agriculture. This characteristic may ensure that T. gamsii YIM PH30019 can exert its biocontrol efficacy in hyperosmotic soil.
Dual culture and induced VOCs assay presented effective antagonism of T. gamsii YIM PH30019 on test pathogenic fungi associated with root-rot diseases of P. notoginseng (Fig. 3, Fig. 4, Fig. 5, Fig. 6). Other T. gamsii isolates also showed antagonistic activity to phytopathogens [39], [40]. The recognition process was thought as the precondition necessary to inhibit the pathogens during the mycoparasitism that happened between Trichoderma and pathogens [41], [42]. The mycoparasitism process of coiling, which depends on recognition, was also detected in both dual culture and SEM observations in our study.
Trichoderma spp. can produce VOCs that inhibit the growth of plant pathogenic fungi via soil air diffusion [43] or induce a defense response in plants [44], [45]. The VOC profiles of Trichoderma species include alcohols, ketones, alkanes, furanes, pyrones, and terpenes [24], which have varying degrees of antagonistic activity against pathogenic fungi. Cocultivation with pathogenic fungus could significantly enrich the metabolites of T. harzianum in comparison to the pure culture [46]. The deactivated pathogenic cell walls could induce T. harzianum to produce greater levels of some proteins [47]. T. gamsii YIM PH30019 produced VOCs induced in the deactivated cell wall medium. These VOCs also presented antagonistic activity to the phytopathogens. However, the T. gamsii antagonism of VOCs induced by pathogenic fungi in the process of biocontrol has been poorly studied. In this study, deactivated cell walls of pathogenic fungi could induce T. gamsii YIM PH30019 to produce different VOC profiles, including dimethyl disulfide, dibenzofuran, methanethiol, and ketones. It is noteworthy that dimethyl disulfide is reported to have favorable antagonistic activity against insects and pathogens associated with many important plants and crops [48], [49]. Many chemical fumigants are used to control plant disease at present [50], but they could change the biological equilibrium [51], such as eradicating beneficial organisms [52] and increasing pathogen populations [53]. Nonchemical methods that effectively control plant diseases are highly desirable. The results give us insight that the deactivated cell walls of pathogenic fungi could be an effective activator for T. gamsii YIM PH30019 to produce more VOCs that inhibit the growth and metabolism of pathogenic fungi. This interesting phenomenon warrants further investigation in ongoing studies.
5. Conclusion
T. gamsii YIM PH30019, isolated as an endophytic fungus, showed no pathogenicity to the host plant of P. notoginseng, and presented favorable biocontrol efficacy in vitro and field evaluation with continuous cropping soil. It could grow well in high concentrations of chemical fertilizers, produce a plenty of VOCs to inhibit the growth of pathogenic fungi, and protect notoginseng against infection by phytopathogens. These results indicate that T. gamsii YIM PH30019 can be used as a promising biocontrol agent against the phytopathogenic fungi of P. notoginseng. In-depth studies should be carried out in different and complex field conditions to evaluate its biocontrol efficacy and influence on indigenous microbial communities, as well as the effect of agro approaches (chemical fertilizers, pesticides, fungicides, etc.) on T. gamsii YIM PH30019.
Conflicts of interest
The authors have no conflicts of interest with any parties or individuals.
Acknowledgments
The authors appreciate Dr Deene Manik Prabhu's kind help and advices to the manuscript. This work was financially supported by the National Natural Science Foundation of China (NSFC, No. 41361075), Key Foundation Program of Yunnan Province of China (No. 2013FA015), Major Program of Educational Commission of Yunnan Province of China (No. ZD2013008), and the Basic Research Foundation of Yunnan University (2013S204).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2015.09.006.
Contributor Information
Hui-Lin Guan, Email: ghl0871@163.com.
Li-Xing Zhao, Email: zlx70@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Dong T.T.X., Cui X.M., Song Z.H., Zhao K.J., Ji Z.N., Lo C.K., Tsim K.W.K. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J Agric Food Chem. 2013;51:4617–4623. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- 2.You C.M., Chen X.B., Tu W., Lou Q., Guan H.L., Xie J. Theoretical thinking about Panax notoginseng's no-tillage cropping soil barriers and mitigation measures. J Yunnan Normal Univ. 2010;30:44–48. [in Chinese] [Google Scholar]
- 3.Wan J.B., Yang F.Q., Li S.P., Wang Y.T., Cui X.M. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J Pharmaceut Biomed. 2006;41:1591–1601. doi: 10.1016/j.jpba.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 4.Liu L., Liu D.H., Jin H., Feng G.Q., Zhang J.Y., Wei M.L., Zhao Z.L. Overview on the mechanisms and control methods of sequential cropping obstacle of Panax notoginseng F.H. Chen. J Mountain Agric Biol. 2011;30:70–75. [in Chinese] [Google Scholar]
- 5.Miao Z.Q., Li S.D., Liu X.Z., Chen Y.J., Li Y.H., Wang Y., Guo R.J., Xia Z.Y., Zhang K.Q. The causal microorganisms of Panax notoginseng root rot disease. Sci Agric Sin. 2006;39:1371–1378. [in Chinese] [Google Scholar]
- 6.Miao C.P., Qiao X.G., Zheng Y.K., Chen Y.W., Xu L.H., Guan H.L., Zhao L.X. First report of Fusarium flocciferum causing root rot of Sanqi (Panax notoginseng) in Yunnan, China. Plant Dis. 2015 [Google Scholar]
- 7.Zhang W., Liao J.J., Zhu G.L., Zhang H., Duan X.L., Zhu S.S., Yang M. The study of inhibitory activity of eight plant volatiles and extracts to Panax notoginseng root rot pathogens. Chinese Agric Sci Bull. 2013;29:197–201. [in Chinese] [Google Scholar]
- 8.Moebius-Clune B.N., Van Es H.M., VanEs O.J., Idowu R.R., Schindelbeck J.M., Kimetu S., Ngoze J. Long-term soil quality degradation along a cultivation chronosequence in western Kenya. Agr Ecosyst Environ. 2011;141:86–99. [Google Scholar]
- 9.Singh J.S., Pandey V.C., Singh D.P. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric Ecosyst Environ. 2015;140:339–353. [Google Scholar]
- 10.Mohamed H.A.L.A., Haggag W.M. Biocontrol potential of salinity tolerant mutants of Trichoderma harzianum against Fusarium oxysporum. Braz J Microbiol. 2006;37:181–191. [Google Scholar]
- 11.Djonovic S., Vargas W.A., Kolomiets M.V., Horndeski M., Wiest A., Kenerley C.M. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007;145:875–889. doi: 10.1104/pp.107.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohmann P., Jones E.E., Hill R.A., Stewart A. Understanding Trichoderma in the root system of Pinus radiata: associations between rhizosphere colonisation and growth promotion for commercially seedlings. Fungal Biol UK. 2011;115:759–767. doi: 10.1016/j.funbio.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Abada K.A. Fungi causing damping-off and root-rot on sugar-beet and their biological control with Trichoderma harzianum. Agric Ecosyst Environ. 1994;51:333–337. [Google Scholar]
- 14.Anees M., Tronsmo A., Edel-Hermann V., Hjeljord L.G., Héraud C., Steinberg C. Characterization of field isolates of “Trichoderma” antagonistic against ‘Rhizoctonia solani’. Fungal Biol UK. 2010;114:691–701. doi: 10.1016/j.funbio.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Verma M., Brar S.K., Tyagi R.D., Surampalli R.Y., Valéro J.R. Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem Eng J. 2007;37:1–20. [Google Scholar]
- 16.Aochi Y.O., Farmer W.J. Impact of soil microstructure on the molecular transport dynamics of 1, 2-dichloroethane. Geoderma. 2005;127:137–153. [Google Scholar]
- 17.Dennis C., Webster J. Antagonistic properties of species-groups of ‘Trichoderma’: II. Production of volatile antibiotics. Trans Br Mycol Soc. 1971;57:41–48. IN4. [Google Scholar]
- 18.Effmert U., Kalderás J., Warnke R., Piechulla B. Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol. 2012;38:665–703. doi: 10.1007/s10886-012-0135-5. [DOI] [PubMed] [Google Scholar]
- 19.Contreras-Cornejo H.A., Macías-Rodríguez L., Herrera-Estrella A., López-Bucio J. The 4-phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant Soil. 2014;379:261–274. [Google Scholar]
- 20.Yang Z., Yu Z., Lei L., Xia Z., Shao L., Zhang K., Li G. Nematicidal effect of volatiles produced by Trichoderma sp. J Asia-pac Entomol. 2012;15:647–650. [Google Scholar]
- 21.Fiers M., Lognay G., Fauconnier M.L., Jijakli M.H. Volatile compound-mediated interactions between barley and pathogenic fungi in the soil. PloS One. 2013;8:e66805. doi: 10.1371/journal.pone.0066805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garbeva P., Hordijk C., Gerards S., De Boer W. Volatiles produced by the mycophagous soil bacterium Collimonas. FEMS Microbiol Ecol. 2014;87:639–649. doi: 10.1111/1574-6941.12252. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F., Yang X., Ran W., Shen Q. Fusarium oxysporum induces the production of proteins and volatile organic compounds by Trichoderma harzianum T-E5. FEMS Microbiol Lett. 2014;359:116–123. doi: 10.1111/1574-6968.12582. [DOI] [PubMed] [Google Scholar]
- 24.Stoppacher N., Kluger B., Zeilinger S., Krska R., Schuhmacher R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J Microbiol Methods. 2010;81:187–193. doi: 10.1016/j.mimet.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Crutcher F.K., Parich A., Schuhmacher R., Mukherjee P.K., Zeilinger S., Kenerley C.M. A putative terpene cyclase, vir4, is responsible for the biosynthesis of volatile terpene compounds in the biocontrol fungus Trichoderma virens. Fungal Genet Biol. 2013;56:67–77. doi: 10.1016/j.fgb.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Yang H.H., Yang S.L., Peng K.C., Lo C.T., Liu S.Y. Induced proteome of Trichoderma harzianum by Botrytis cinerea. Mycol Res. 2009;113:924–932. doi: 10.1016/j.mycres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Rothrock C.S. Take-all of wheat as affected by tillage and wheat soybean doublecropping. Soil Biol Biochem. 1987;19:307–311. [Google Scholar]
- 28.Huang X., Chen L., Ran W., Shen Q., Yang X. Trichoderma harzianum strain SQR-T37 and its bio-organic fertilizer could control Rhizoctonia solani damping off disease incucumber seedlings mainly by the mycoparasitism. Appl Microbiol Biot. 2011;91:741–755. doi: 10.1007/s00253-011-3259-6. [DOI] [PubMed] [Google Scholar]
- 29.Gao S., Sosnoskie L.M., Cabrera J.A., Qin R., Hanson B.D., Gerik J.S., Wang D., Browne G.T., Thomas J.E. Fumigation efficacy and emission reduction using low-permeability film in orchard soil fumigation. Pest Manag Sci. 2015 doi: 10.1002/ps.3993. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y.Q., Yang L., Wei M.L., Huang T.W. Effects of different treatments and GA3 concentration on induction seedling of Panax notoginseng. Spec Wild Econ Anim Plant Res. 2013;04:47–49. [Google Scholar]
- 31.Paz Z., Gerson U., Sztejnberg A. Assaying three new fungi against citrus mites in the laboratory, and a field trial. Biocontrol. 2007;52:855–862. [Google Scholar]
- 32.Spiegel Y., Chet I. Evaluation of Trichoderma spp. as a biocontrol agent against soilborne fungi and plant-parasitic nematodes in Israel. Integr Pest Manage Rev. 1998;3:169–175. [Google Scholar]
- 33.Mukherjee M., Mukherjee P.K., Horwitz B.A., Zachow C., Berg G., Zeilinger S. Trichoderma–plant–pathogen interactions: advances in genetics of biological control. Indian J Microbiol. 2012;52:522–529. doi: 10.1007/s12088-012-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viterbo A., Landau U., Kim S., Chernin L., Chet I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol Lett. 2010;305:42–48. doi: 10.1111/j.1574-6968.2010.01910.x. [DOI] [PubMed] [Google Scholar]
- 35.Morath S.U., Hung R., Bennett J.W. Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol Rev. 2012;26:73–83. [Google Scholar]
- 36.Zhang Z.L., Wang W.Q., Yang J.Z., Cui X.M. Effects of continuous Panax notoginseng cropping soil on P. notoginseng seed germination and seedling growth. Soils. 2010;42:1009–1014. [in Chinese] [Google Scholar]
- 37.Turco E., Vizzuso C., Franceschini S., Ragazzi A., Stefanini F.M. The in vitro effect of gossypol and its interaction with salts on conidial germination and viability of Fusarium oxysporum sp. vasinfectum isolates. J Appl Microbiol. 2007;103:2370–2381. doi: 10.1111/j.1365-2672.2007.03503.x. [DOI] [PubMed] [Google Scholar]
- 38.El-Abyad M.S., Hindrof H., Rizk M.A. Impact of salinity stress on soil-borne fungi of sugarbeet: II. Growth activities in vitro. Plant Soil. 1988;110:33–47. [Google Scholar]
- 39.Aydin M.H., Turhan G. The efficacy of Trichoderma species against Rhizoctonia solani in potato and their integration with some fungicides. Anadolu. 2013;23:12–31. [Google Scholar]
- 40.Gilardi G., Demarchi S., Gullino M.L., Garibaldi A. Nursery treatments with non-conventional products against crown and root rot, caused by Phytophthora capsici, on zucchini. Phytoparasitica. 2015 [Google Scholar]
- 41.Benhamou N., Chet I. Hyphal interactions between Trichoderma harzianum and Rhizoctonia solani: ultrastructure and gold cytochemistry of the mycoparasitic process. Phytopathology. 1993;83:1062–1071. [Google Scholar]
- 42.López-Mondéjar R., Antón A., Raidl S., Ros M., Pascual J.A. Quantification of the biocontrol agent Trichoderma harzianum with real-time TaqMan PCR and its potential extrapolation to the hyphal biomass. Bioresource Technol. 2010;101:2888–2891. doi: 10.1016/j.biortech.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Minerdi D., Bossi S., Gullino M.L., Garibaldi A. Volatile organic compounds: a potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environ Microbiol. 2009;11:844–854. doi: 10.1111/j.1462-2920.2008.01805.x. [DOI] [PubMed] [Google Scholar]
- 44.Ryu C.M., Farag M.A., Hu C.H., Reddy M.S., Wei H.X., Paré P.W., Kloepper J.W. Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishimoto K., Matsui K., Ozawa R., Takabayashi J. Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J Gen Plant Pathol. 2007;73:35–37. [Google Scholar]
- 46.Vinale F., Ghisalberti E.L., Sivasithamparam K., Marra R., Ritieni A., Ferracane R., Woo S., Lorito M. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett Appl Microbiol. 2009;48:705–711. doi: 10.1111/j.1472-765X.2009.02599.x. [DOI] [PubMed] [Google Scholar]
- 47.Tseng S.C., Liu S.Y., Yang H.H., Lo C.T., Peng K.C. Proteomic study of biocontrol mechanisms of Trichoderma harzianum ETS 323 in response to Rhizoctonia solani. J Agr Food Chem. 2008;56:6914–6922. doi: 10.1021/jf703626j. [DOI] [PubMed] [Google Scholar]
- 48.Dugravot S., Grolleau F., Macherel D., Rochetaing A., Hue B., Stankiewicz M., Huignard J., Lapied B. Dimethyl disulfide exerts insecticidal neurotoxicity through mitochondrial dysfunction and activation of insect KATP channels. J Neurophysiol. 2003;90:259–270. doi: 10.1152/jn.01096.2002. [DOI] [PubMed] [Google Scholar]
- 49.Kyung K.H., Fleming H.P. Antimicrobial activity of sulfur compounds derived from cabbage. J Food Prot. 1997;60:67–71. doi: 10.4315/0362-028x-60.1.67. [DOI] [PubMed] [Google Scholar]
- 50.Li Y., Mao L., Yan D., Ma T., Shen J., Guo M., Wang Q., Ouyang C., Cao A. Quantification of Fusarium oxysporum in fumigated soils by a newly developed real-time PCR assay to assess the efficacy of fumigants for Fusarium wilt disease in strawberry plants. Pest Manag Sci. 2014;70:1669–1675. doi: 10.1002/ps.3700. [DOI] [PubMed] [Google Scholar]
- 51.Gu Y.Q., Mo M.H., Zhou J.P., Zou C.S., Zhang K.Q. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol Biochem. 2007;39:2567–2575. [Google Scholar]
- 52.Spath M., Insam H., Peintner U., Kelderer M., Kuhnert R., Franke-Whittle I.H. Linking soil biotic and abiotic factors to apple replant disease: a greenhouse approach. J Phytopathol. 2015;163:287–299. [Google Scholar]
- 53.Gamliel A., Austerweil M., Kritzman G. Non-chemical approach to soilborne pest management–organic amendments. Crop Prot. 2000;19:847–853. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.