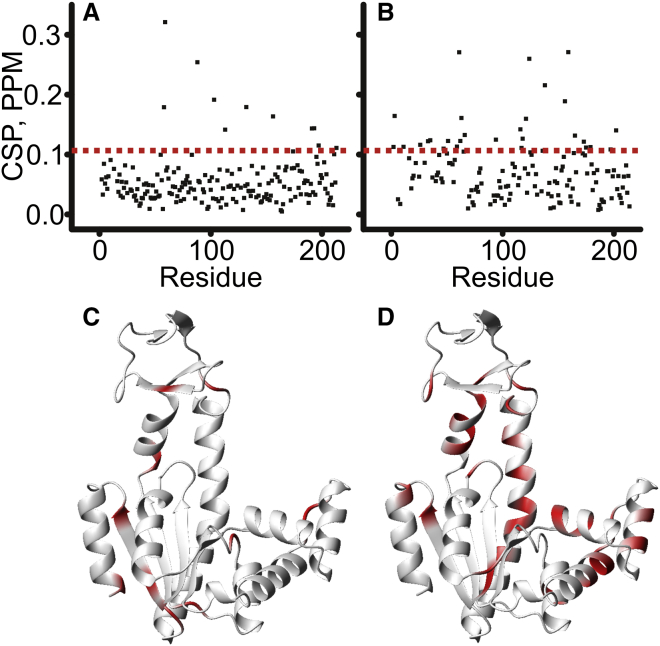
Figure 11.
Urea-dependent chemical shift perturbations in Adk in the apo state (A) and bound to Ap5A (B) between 0 and 2.5 M urea. The compounded 1H and 15N chemical shift changes were calculated from (∣ΔωH∣+∣ΔωN∣∗0.2). (C and D) Structural display of the amino acid residues with chemical shift perturbations larger than 1 SD above the average (indicated by the dotted line in A and B) are colored in red for Apo Adk (C) and Ap5A-bound Adk (D). Both data sets are visualized on the open (Apo) structure (11) for clarity.