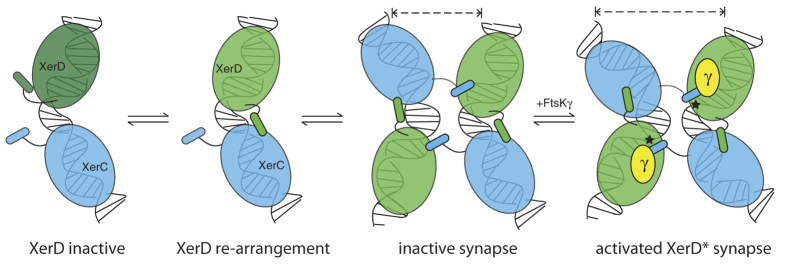
Figure 6. Schematic model of recombination.
XerC (blue) and XerD (green) bind to the two halves of the dif site. Initially XerD is in the inactive state (dark green) as seen in the crystal structure (Fig. 1). Upon re-modelling of the three C-terminal helices of XerD as described, the active site is now close to the cleavage competent state (depicted by light green XerD) and the very C-terminal helix (helix N) rotates so that it can now interact with the XerC binding partner. At synapsis, two XerCD-dif sites come together and the potential for a pseudo-fourfold symmetric arrangement of interactions is present, with the N-helices of XerC stretching across synaptic partners to the neighbouring XerD monomers. Upon interaction of FtsKγ there is a modest re-modelling of the complex to increase the bending of the DNA. The FtsKγ domain interacts above the cleft in XerD in which the XerC N-helix sits. Only when all these conditions are achieved does XerD become catalytically active, as denoted by the black asterisk.