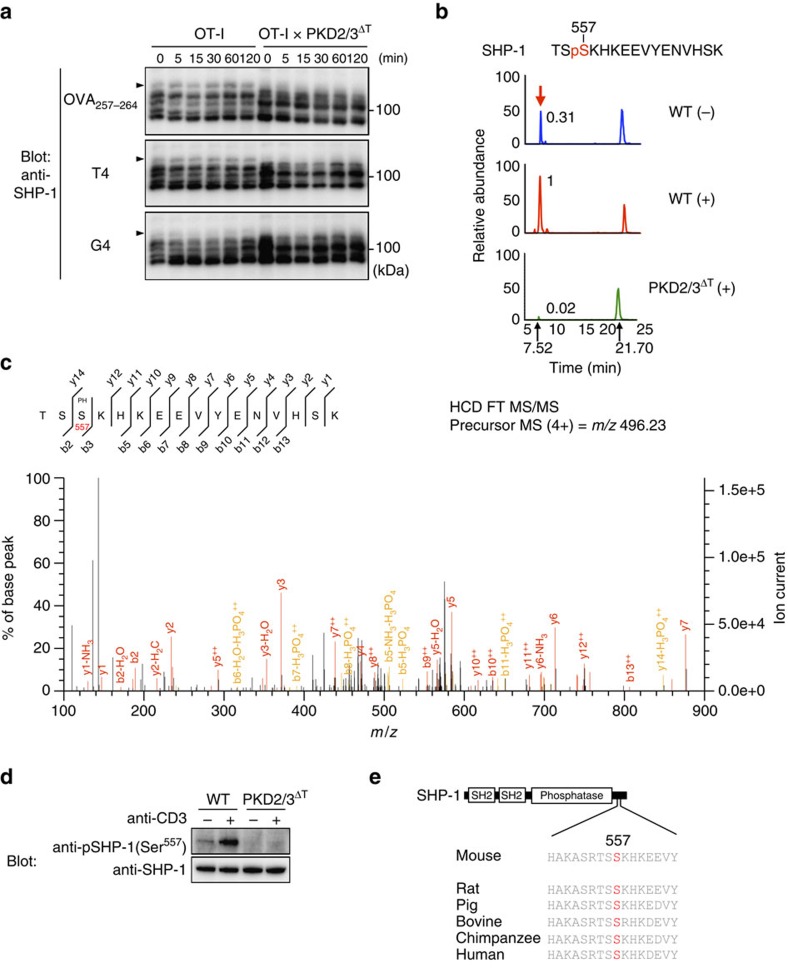
Figure 9. SHP-1 is phosphorylated by PKD2 and PKD3 at Ser557 in thymocytes.
(a) Phosphorylation of SHP-1 on peptide stimulation. OT-I DP thymocytes were stimulated with the designated OVA peptides for the indicated times. Cell lysates were subjected to Phos-tag immunoblot analysis using anti-SHP-1. Arrowheads indicate phosphorylated SHP-1 that showed stimulation-dependent mobility shift. (b) The extracted ion chromatogram of m/z 496.23096 corresponds to the quadruply charged SHP-1 phosphopeptide (TSpS557KHKEEVYENVHSK), which was increased by TCR stimulation in tryptic phosphopeptides from WT thymocytes and was almost undetectable in those from stimulated PKD2/3ΔT thymocytes. The relative values of the peak area are indicated. Note that the hextuply charged ion peak at a retention time of 21.70 min was unrelated to this SHP-1 phosphopeptide. (c) Phosphorylation of Ser557 was demonstrated by the MS/MS spectrum of the m/z 496.23 ion at a retention time of 7.52 min in tryptic phosphopeptides from stimulated-WT thymocytes. (d) Phosphorylation of Ser557 of SHP-1 was analysed by immunoblotting. Total thymocytes were stimulated by CD3 cross-linking for 2 min and analysed by anti-phospho-SHP-1 (Ser557) Abs. Total SHP-1 was blotted as a control. (e) Schematic structure of SHP-1 and sequence alignment of the C-terminal region of SHP-1 that contains Ser residue corresponding to Ser557 of murine SHP-1 (shown in red). Data are representative of two independent experiments (a–d).