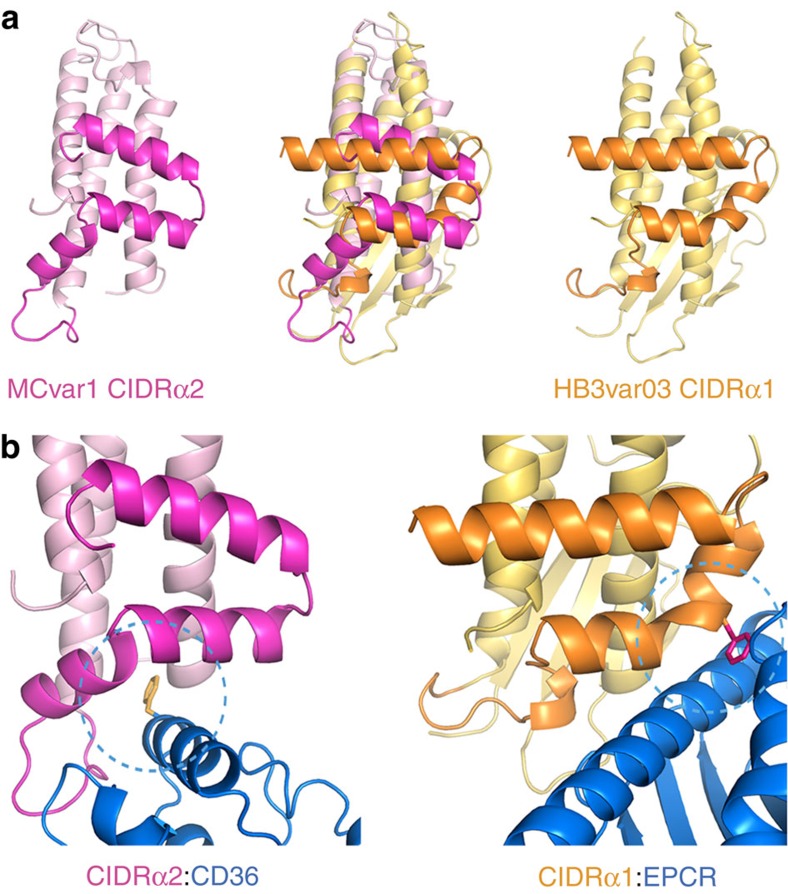
Figure 3. A comparison of the features that allow CIDRα domains to bind to EPCR or CD36.
(a) A comparison of the structure of a CD36-binding CIDRα2 domain (pink) with that of an EPCR-binding CIDRα1 domain (orange). Both domains share a core three α-helical bundle. The insertion that emerges between the second and third of these core helices forms a docking platform for ligands. (b) A close up of the binding interfaces that mediates the CIDRα2:CD36 and CIDRα1:EPCR interactions. The CIDRα1 domains have a phenylalanine residue (F656) on a convex surface of the domain that protrudes into the hydrophobic groove of EPCR. In contrast, the CIDRα2 domains have a hydrophobic pocket that binds to a protruding phenyalanine residue (F153) from CD36.