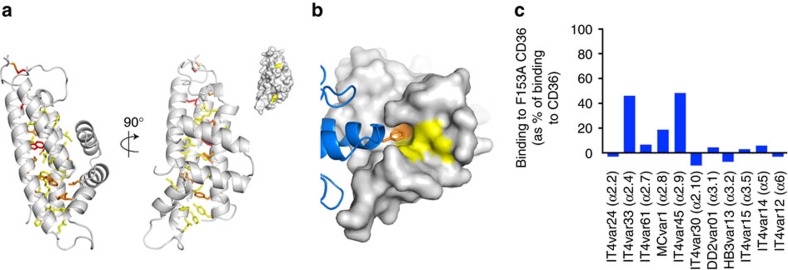
Figure 5. Limited chemical conservation allows CD36 binding.
(a) Conservation in the CD36-binding CIDRα domain is plotted onto the structure of the MCvar1 CIDRα2.8 domain. Absolutely conserved residues are shown as red sticks. Residues with property entropy score of less than 0.1 (but not totally conserved) are orange and those with scores of 0.1–0.3 are yellow. The inset shows a surface representation in the same orientation and colours, showing that conserved residues cluster predominantly in core of the domain, stabilizing its structure. (b) A surface representation of the CIDRα domain coloured as in A, with CD36 in blue. This shows that residues in the hydrophobic pocket of the CIDRα domain are the most chemically conserved feature on the CIDRα domain surface. (c) The effect of the F153A mutant of CD36 on the binding of a diverse panel of CIDRα2-6 domains shows that the interaction mediated by F153 of CD36 plays an important role in binding across the CIDRα2-6 domain family.