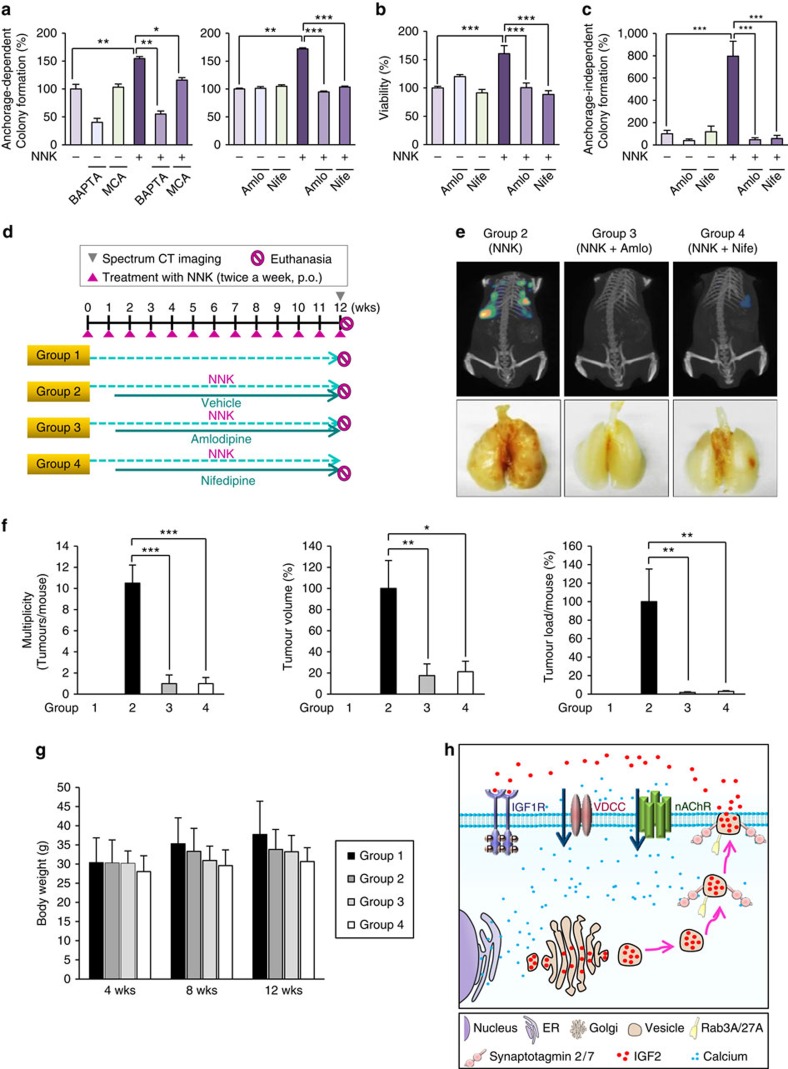
Figure 6. Suppression of NNK-induced acquisition of transformed phenotypes in vitro and lung tumour formation in vivo by blockade of Ca2+ influx.
(a–c) Anchorage-dependent colony formation (n=3, mean±s.d.) (a), cell viability (n=3, mean±s.d.) (b), and anchorage-independent colony formation (n=4, mean±s.d.) (c) of BEAS-2B cells treated with NNK in combination with BAPTA-AM (BAP), mecamylamine (MCA), nifedipine (Nife), or amlodipine (Amlo). *P<0.05; **P<0.01; ***P<0.001, Student's t-test. (d) Schematic representation of NNK and CCBs (amlodipine and nifedipine) treatment schedule. Mice were treated with NNK (n=10) alone or in combination with amlodipine (n=10) or nifedipine (n=10) for 12 weeks. (e) Top: representative IVIS Spectrum CT images of mice treated with NNK (group 2), NNK and amlodipine (group 3), or NNK and nifedipine (group 4). Bottom: representative images of the lung from groups 2, 3 and 4. (f) Tumour multiplicity, volume, or load of groups 1, 2, 3 and 4 at 12 weeks of treatment (n=6, mean±s.d.). *P<0.05; **P<0.01; ***P<0.001, Student's t-test. (g) Body weight changes in groups 1 (Control), 2 (NNK), 3 (NNK+amlodipine) and 4 (NNK+nifedipine) at 4, 8 and 12 weeks of treatment. (h) Schematic model for the NNK-induced lung tumorigenesis via regulation of the IGF-1R signalling by the nAChR-VDCC-mediated calcium influx. In light of our present findings, NNK can increase intracellular Ca2+ level via α7nAChR and VDCC, leading to IGF2 secretion by exocytosis. Syt2, Syt7, Rab3A and Rab27A are thought to play a major role in NNK-induced exocytosis of IGF2. Finally, secreted IGF2 can activate IGF-1R signalling that promotes lung cancer formation.