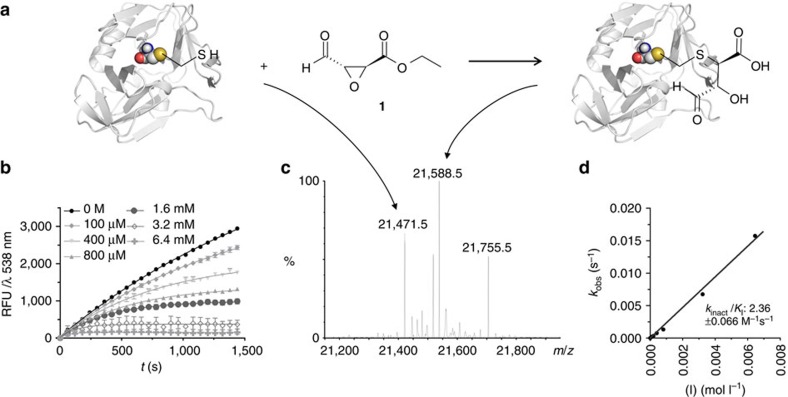
Figure 2. Development of the bis-electrophilic warhead 1.
(a) Deactivation of Coxsackie virus 3C protease by reaction with bis-electrophilic warhead 1. Reaction scheme. (b) Inhibition of the protease in the FRET-based enzyme activity assay at various concentrations of 1. (c) Deconvoluted ESI-mass spectrum of the reaction of the protease with 1. The mass of the unmodified protein, 21,471.5 Da, is shifted to a main peak at 21,588.5 Da, corresponding to the mass of the protein +1 after cleavage of the ethyl ester. The peak at 21,755.5 corresponds to the protein mass +1 +Tris (buffer)–water. (d) Deactivation of the protease is quantified as relative inhibition, the observed deactivation rate, kobs, plotted against inhibitor concentration. The slope of the linear regression curve of the kobs values plotted against the corresponding concentration value yields the kinact/KI-value for compound 1 and equals 2.4±0.1 M−1 s−1.